Abstract
The objective of the present study was to monitor and characterize morphological alterations in ovaries of agouti (Dasyprocta prymnolopha), reared in captivity, by using abdominal ultrasonography. All animals underwent daily vaginal cytological examination to identify the current cycle phase. For each phase of the estrous cycle, ultrasound examinations were carried out to identify and describe the morphology of both ovaries. Topographic parameters in an ultrasound window were established to locate the ovaries. The agouti estrous cycle lasted an average of 29.94 ± 6.77 days. During vaginal cytology examinations, all cell types were identified, and each phase of the estrous cycle was established by cell counts. No significant alterations were observed in the assessed ovarian morphometry measurements. In 75% of the animals examined, ovarian follicle presence was observed in the proestrus phase.
In the past, agricultural activities were restricted to providing human subsistence, but with the development of societies, searching for agricultural improvement and professionalization became necessary for successful animal rearing. For greater success in rearing traditional animals, such as cattle, pigs, goats, and sheep, adequate rearing conditions were needed, and such conditions depended on diverse factors such as climate, biological conditions, and production seasonality [22].
Rearing native wild animals may take the place of traditional cattle raising for low-income rural producers in regions that are less suitable for more demanding rearing practices, such as those associated with cattle raising and poultry farming. That alternative is already a reality [17], especially in countries such as Brazil where hunting is not allowed and access to wild animal meat is obtained clandestinely. Such wild animal rearing practices indirectly help the conservation of the species in nature, because they provide an established legal market for animals born in captivity and raised for a predetermined purpose, potentially ending the practice of illicit hunting in countries where hunting is not permitted [11].
However, as in every type of animal rearing and management, wild animal production systems need improvement because little has been reported about the proper reproduction and production management of wild animals; such information would enable rational, large-scale production, helping to produce a source of low-cost animal protein [4,13,15].
Among the wild animals with potential for production management, agoutis of the genus Dasyprocta are rodents that have been shown to have acceptable reproductive indices in captivity [8], and such results have triggered interest in research related to agouti production. Some data on the reproduction of agoutis have been published, and previous study has shown that the duration of the agouti estrous cycle varies according to the species, with an average cycle period of 30 days in Dasyprocta prymnolopha [8], 28 days in Dasyprocta leporina [1,2], and 34 days in Dasyprocta aguti [23]. These studies were based on vaginal cytology, but the use of an easy, fast, non-invasive technique, such as ultrasonography, may be an effective tool for monitoring and increasing the understanding of the reproductive physiology of various animals. Both ultrasonography and vaginal cytology are viable alternatives for identifying the presence of pre-ovulatory follicles in agouti and for developing indices that are useful in determining estrus [2].
Although previous agouti studies have enriched human knowledge, they have not yet sufficiently described agouti reproduction to guarantee adequate reproduction of this species either in captivity or the wild [20]; thus, further investigations into the reproductive aspects of the agouti are needed. With the perspective of elucidating the reproductive physiology of D. prymnolopha, the present study aimed to characterize the modifications in the ovarian morphology of this species during the different phases of the estrous cycle by using ultrasonography. In addition, the study aimed to obtain descriptive ultrasound data on ovary morphology and topography in loco.
Agoutis are members of the Mammalian class and are in the order Rodentia, suborder Hystricomorpha, infraorder Hystricognathi, family Dasyproctidae, and genus Dasyprocta. They are land animals that have thin legs compared to their trunk. They are extremely agile, have a rough, uniformly ochre-colored coat with orange to red tones, and have diurnal and crepuscular habits. [10]. Adult agouti average 50 cm long, from snout to tail base, and are approximately 23 cm tall and weigh between 2 and 3 kg. They are reported to live for 8 to 10 years [5].
Eleven healthy D. prymnolopha (ten females and one vasectomized male) were included in the study. They were obtained from the Nucleus for Wild Animal Preservation (NEPAS) located at the Federal University of Piaui, Brazil. The experiments were carried out in the Laboratory of Morphological Research at the Federal University of Campina Grande, Patos, Paraíba, Brazil. The methodological protocols of the project were approved by the Ministry of the Environment and by the Biodiversity Authorization and Information System of the Chico Mendes Biodiversity Conservation Institute (protocol No. 45046-1 and 47944-1), and by the Committee of Ethics in Animal Use of the Research Ethics Commission (CEP N° 237–2014) of the Federal University of Campina Grande.
The animals were kept in a box with a 24 m2 covered area supplied with natural light and ventilation. The composition of the floor was mixed, part concrete (16 m2) and part sand (8 m2), there was environmental enrichment in the form of masonry dens/hutches and plants. Feed was provided as an extruded meal consisting of 13% moisture, 14% crude protein, 15% acid detergent fiber, 0.6% phosphorus, 15% fibrous matter, 17% mineral material, 4% ether extract, and 2% calcium.
The animals were kept together, simulating a family environment, with the vasectomized male present to promote sexual stimulation without the risk of pregnancy. There was a one-month adaptation period before the experiments started. An electronic surveillance system with a high-resolution camera (Infra model; Jortan, Brazil) coupled to a hard disk (model H.264; Jortan) to monitor the animals' behavior. After confirming the presence of behaviors, such as foraging with the front paws, resting, lying on top of the limbs, and self-directed care such as licking the back and limbs, that were deemed indicative of comfort with and adaptation to their environment [12], the experiments were started. In the experiments, the individual phases within two complete estrous cycles of each female were monitored by obtaining daily vaginal cytology samples via sterile swab imbibed in 0.9% physiological solution inserted into the vagina. Cytology smear slides were prepared for examination under a light microscope. Estrous cycle phases were identified based on the cell count, which involved counting 100 cells per site and identifying the large and small parabasal, intermediary, nucleated, and anucleated surface cells and determining their proportions, according to a methodology previously described [8]. Furthermore, the anatomic characteristics of the external genitalia of the animals were observed and recorded before starting the cytological sampling [8].
Whenever a change in estrous cycle phase was identified by the vaginal cytology results, the animals were submitted to a food fast of six hours, due to the high mobility of their intestinal loops, and then sent to the Laboratory of Morphological Research at the Federal University of Campina Grande. At the laboratory, the animals were restrained manually in lateral decubitus, the lateral abdominal regions were shaved, and the animals were submitted to ultrasonographic examination by using the model Z5 Vet ultrasound apparatus (Mindray, China) attached to a linear transductor at 7.5 Hz frequency. Each ovary was located by assigning the kidney as topographical reference in the caudal region, and the ovary suspensory ligament and the ovarian artery as dorsal and ventral topographical references, respectively, and then establishing a topographic triangle of the three reference locations to facilitate ovary identification. Each ovary was measured to determine the largest and smallest diameters and their respective areas, as well as their morphological echotexture and echogenicity, were determined. When identification was possible, follicles were counted and measured.
Statistical assessment of ovary measurement mean values was carried out by using InStat 3 software (GraphPad, USA) and included analysis of variance and comparison of means by applying the Tukey test; statistical significance was set at the 5% level (p < 0.05).
The estrous cycle of the 10 examined female agoutis had a mean duration of 29.94 ± 6.77 days with an 18 to 41 day range (Table 1). The anatomic characteristics of the agouti external genitalia showed alterations such as an increase in volume, the presence of hyperemia around the urogenital region, cervical canal narrowing, and presence of mucus with a characteristic odor.
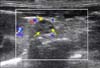
For improved ultrasonography, there was a need for previous fasting from food and liquid ingestion in order to reduce the quantity of gas inside the intestinal loops. It was then possible to standardize ultrasonographic access to the agouti ovary through the lateral abdominal region and in the right and left paralumbar fossa, resulting in a window formed by the image of the respective kidney and using the caudal edge of the kidney cranially, the ovary suspensory ligament dorsally, and the image of blood flow (captured by using color Doppler) in the ovarian vein and artery ventrally as topographical references. A triangle was formed from these three reference points and the right or left ovary was located at the center and upper right vertex of the same-side triangle (Fig. 1).
Both left and right ovaries presented as flattened, hypoechoic structures that were similar morphometrically. The right ovary presented in a more unstable location than the left ovary because of the strong influence of intestinal peristalsis that hindered right ovary localization during ultrasonographic examination. The ovary measurements for each phase of the estrous cycle showed no significant differences in diameter or area for either of the ovaries (p > 0.05) (Table 2). Reproductive structures, such as the ovarian follicles, were identified in the ovarian parenchyma in 75% of the ovaries assessed; this low percentage is because of difficult delimitation and the ultrasonographic similarity of follicles and ovarian parenchyma (Fig. 2).
All the follicles identified were observed to be in the proestrus phase according to morphometric measurements indicative of pre-ovulatory follicles (Table 3). Fourteen ovarian follicles were observed in 75% of the ovaries examined.
Notably, follicular blood flow could not be visualized by using color Doppler ultrasonography; perhaps because their discreet circulation was not perceptible by the apparatus used in this study. In addition, it was not possible to identify the corpus luteum by ultrasonography in the present study. Interestingly, there was no need to restrain the animals chemically to carry out the examinations; that characteristic makes them practical, safe, and economical for large-scale production management because manual restraint was adequate for carrying out ultrasonography.
After the adaptation period, the animals showed stable behavior, a characteristic of agouti in captivity. Some actions were performed in repose such as movements of the head and snout in response to the environment; also body movements with thoracic limbs used to alter aspects of the surroundings, mainly those related to looking for and keeping food [12]. These behaviors served as a reference to assess the animals' adaptability to their environment and to ensure that no behavioral factor was interfering with their reproductive physiology; the lack of such interference was demonstrated by the results showing that the duration of the estrous cycle was similar to the mean duration for the genus [28]. Corroborating data obtained by other researchers [1011] has shown that estrous cycle duration is species-dependent but varies from one female to another within the same species. Although confined in the same environment, synchronization of the estrous cycles, due to a male effect among the females, was not observed in this study, although it has been reported in other studies on reproductive management in domestic animals [141619].
Several morphologically different cell layers formed the vaginal mucosa. These layers vary in thickness during the estrous cycle as well as during gestation, anestrus, and lactation [7]. Under estrogenic action, the layers of the vagina stratified epithelium (especially the most superficial layers) proliferate, resulting in exfoliation of characterized keratinized cells. However, when progesterone effects predominate and there is low estrogenic activity, the epithelial cells present in the deeper layers proliferate and mature [21]. In the cytological examinations carried out on the agoutis in this study, all cell types could be observed in all phases of the estrous cycle. The phases were identified by determining the proportional quantity of each cell type at the different phases, following the methodology previously described for Dasyprocta [8]. In contrast, another study [2] indicated that proestrus could not be distinguished from estrus by using vaginal cytology in D. leporina. In a study carried out on wild peccary, Pecari tajacu, a species native to Brazil [9], cell standardization that was similar to that in the present study was reported. Cell morphology and cell type in each phase of the estrous cycle are highlighted by a marked presence of surface and intermediate cells. To differentiate each phase requires the presence or absence of leucocytes, which are associated with changes in the characteristics of the external genitalia, as was observed in the individuals in the present study. We emphasize that the external genitalia characteristics were not well defined in some animals, even though they were at the estrus phase as determined by vaginal cytology.
With regard to ultrasound examinations carried out on individuals of the genus Dasyprocta, few studies [2] have reported a standardized approach using topographic points to derive an exact ovary location and assess ovarian morphology. In addition, structures adjacent to the ovaries can hinder the use of that technique in individuals of the same genus.
Previous fasting from food and avoidance of liquid ingestion to reduce the quantity of gases in intestinal loops allows better ultrasonic assessment. This is similar to the procedures indicated for small animals, demonstrating that, despite specific anatomic characteristics, visualization in agoutis is subject to factors similar to those presented by peristalsis in domestic animals [3]. In small animals, during the proestrus phase, because of the influence of estrogen, the reproductive tract is altered, showing an increase in volume [3]. In the agouti, this type of variation was not detected in the ultrasonography results, leading us to question whether the hemodynamic peculiarities present in D. prymnolopha, and observed in individual D. leporina [2] and Cuniculus paca [6] are factors in revealing flattened structures with hypoechoic density. Regarding the low efficacy of revealing follicle organization and delimitation by ultrasonography, this may be because these animals present ovaries that are covered by a mesosalpinx, specifically in the mesovarium region and on the side surface. Similar observations have been made by other authors in individuals C. paca [18] and D. leporina [212].
The ultrasound-derived ovarian measurements did not provide a standard by which estrous cycle phases could be differentiated because there were no significant differences in length, width, or area of the ovaries among the different phases. However, the ultrasound technique was efficient in identifying and distinguishing the follicular phase. Complimentary studies, such as vaginal cytological and sex hormone dosage studies, are needed to obtain more precise data on the reproductive cycle of this species.
Figures and Tables
Fig. 1
Color Doppler ultrasound ovary image (A) indicating the topographic triangle (B) formed by the caudal edge of the left kidney (1), the ovary suspensory ligament (2), and the ovarian artery (3). At the center and indicated by the arrows are the left ovary of agouti n°437 (OV E 437) (A/B).

Acknowledgments
The authors thank the Coordination for the Improvement of Higher Education Personnel for funding this research, Dr. Paulo Marques Costa, Coordinator of the Study Center and Research on Wild Animals (Nucleus for Wild Animal Preservation) of the Federal University of Piaui, for ceding the animals for the study. We also thank the Laboratory of Animal Reproduction of the Federal University of Campina Grande, which provided us with the equipment used in the examinations.
References
1. Almeida MM, Carvalho MAM, Cavalcante Filho MF, Miglino MA, Menezes DJA. Morfological and morfometric study of the ovary in agoutis (Dasyprocta aguti, Linnaeus, 1766). Braz J Vet Res Anim Sci. 2003; 40:55–62. Portuguese.
2. Campos LB, Peixoto GCX, Lima GL, Castelo TS, Souza ALP, Oliveira MF, Silva AR. Monitoring of the estrous cycle of agoutis (Dasyprocta leporina Lichtenstein, 1823) by vaginal exfoliative cytology and ultrasonography. Pesqui Vet Bras. 2015; 35:188–192. Portuguese.

3. Carvalho CF. Ultrasound in Small Animals. 5th ed. Roca: São Paulo;2004. p. 227–280.
4. Cavalcante RR, Almeida MM, Moura SG, Martins Júnior LM, Conde Júnior AM, Carvalho MAM, Lopes JB. Postpartum weight, frequency and prevalence of the type of calf birth (Dasyprocta sp.) grown in captivity. Ciênc Anim Bras. 2005; 6:67–70. Portuguese.
5. Deustsch LA, Puglia LRR. The Wild Animals: Protection, Dieses and Management. 1st ed. Globo: Rio de Janeiro;1988. p. 45–50. Portuguese.
6. Feliciano MAR, Barros FFPC, Coutinho LN, Brito MBS, Uscategui RR, Santos VJC, Almeida VT, Kawanami AE, Nociti RP, Machado MRF, Vicente WRR. Conventional and Doppler abdominal ultrasonography in pacas (Cuniculus paca). Acta Sci Vet. 2014; 42:1–6.
7. Ghannam SA, Bosc MJ, Du Mesnil du Buisson F. Examination of vaginal epithelium of the sheep and its use in pregnancy diagnosis. Am J Vet Res. 1972; 33:1175–1185.
8. Guimarães DA, Moreira D, Vale WG. Determination of agouti (Dasyprocta prymnolopha) reproductive cycle by colpocytologyc diagnostic. Acta Amaz. 1997; 27:55–64.
9. Guimarães DAA, Garcia SCG, Le Pendu Y, Albuquerque NI. Determination of the estrous cycle in collared peccary Pecari tajacu: colpocytological and clinical aspects. Acta Amaz. 2011; 41:583–588. Portuguese.
10. Hafez ESE, Hafez B. Reproduction in Farm Animals. 7th ed. Manole: São Paulo;2004. p. 55–62. Portuguese.
11. Hosken FM, Silveira AC. Creation of Agoutis. 4th ed. Aprenda Fácil: Viçosa;2001. p. 231.
12. Kaiser SK, Margarido TCC, Fischer ML. Behavioral evaluation of captive and semi-captive agoutis Dasyprocta azarae and Dasyprocta leporina (Rodentia: Dasyproctidae), on urban parks of Curitiba, Paraná, Brazil. Rev Etol. 2011; 10:68–82.
13. Lopes JB, Cavalcante RR, Almeida MM, Carvalho MAM, Moura SG, Dantas Filho LA, Conceição WLF. Performance of agouti (Dasyprocta prymnolopha) bred in captivity according to sex and parturition in Teresina, Piauí. Rev Bras Zootec. 2004; 33:Suppl 3. 2318–2322. Portuguese.
14. Martin GB, Scaramuzzi RJ, Lindsay DR. Effect of the introduction of rams during the anoestrous season on the pulsatile secretion of LH in ovariectomized ewes. J Reprod Fertil. 1983; 67:47–55.

15. Mendonça IL, Almeida MM, Conde Júnior AM, Cavalcante AR, Moura SG, Carvalho MAM. Coproparasitological analysis of agouti (Dasyprocta sp.) in captivity. Ciênc Anim Bras. 2006; 7:285–288. Portuguese.
16. Murata K, Tamogami S, Itou M, Ohkubo Y, Wakabayashi Y, Watanabe H, Okamura H, Takeuchi Y, Mori Y. Identification of an olfactory signal molecule that activates the central regulator of reproduction in goats. Curr Biol. 2014; 24:681–686.

17. Nogueira Filho SLG, Nogueira SSC. Commercial breeding of wild animals: production and marketing of meat and by-products in the southeastern region of Brazil. Rev Econ Nordeste. 2000; 31:188–195. Portuguese.
18. Reis ACG, Gerbasi HB, Martins C, Machado MRF, Oliveira CA. Morphology of the female genital system of the paca (Cuniculus paca, Linnaeus, 1766). Braz J Vet Res Anim Sci. 2011; 48:183–191.
19. Rekwot PI, Ogwu D, Oyedipe EO, Sekoni VO. The role of pheromones and biostimulation in animal reproduction. Anim Reprod Sci. 2001; 65:157–170.

20. Rodrigues RF, Miglino MA, Ferraz RHS, Morais-Pinto L. Placentation in agouti (Dasyprocta aguti, CARLETON, M.D.): morphologic aspects. Braz J Vet Res Anim Sci. 2003; 40:133–137. Portuguese.
21. Schutte AP. Canine vaginal cytology. II. Cyclic changes. J Small Anim Pract. 1967; 8:307–311.
22. Serviço Brasileiro de Apoio ás Micro e Pequenas Empresas (SEBRAE). Starting point for the start of business: creation of agouti. Brasília: SEBRAE;2006.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download