Abstract
To provide insights into the role of innate immune responses in vaccine-mediated protection, we investigated the effect of Marek's disease (MD) vaccine, CVI988/Rispens, on the expression patterns of selected genes associated with activation of macrophages in MD-resistant and MD-susceptible chicken lines. Upregulation of interferon γ, interleukin (IL)-1β, IL-8, and IL-12 at different days post-inoculation (dpi) revealed activation of macrophages in both chicken lines. A strong immune response was induced in cecal tonsils of the susceptible line at 5 dpi. The highest transcriptional activities were observed in spleen tissues of the resistant line at 3 dpi. No increase in the population of CD3+ T cells was observed in duodenum of vaccinated birds at 5 dpi indicating a lack of involvement of the adaptive immune system in the transcriptional profiling of the tested genes. There was, however, an increase in the number of macrophages in the duodenum of vaccinated birds. The CVI988/Rispens antigen was detected in the duodenum and cecal tonsils of the susceptible line at 5 dpi but not in the resistant line. This study sheds light on the role of macrophages in vaccine-mediated protection against MD and on the possible development of new recombinant vaccines with enhanced innate immune system activation properties.
Marek's disease virus (MDV), a highly cell-associated oncogenic α-herpesvirus, is the etiological agent of Marek's disease (MD), a contagious lymphoproliferative disease of domestic chickens [10]. MDV causes immunosuppression, neurological disorders, chronic wasting, blindness, and fatal T cell lymphomas in susceptible chickens [11]. The early pathogenesis of MD is associated with an initial lytic infection of B cells followed by latent infection of activated CD4+ T cells that leads to the destruction of B and T cells, atrophy of bursa of Fabricius and thymus, and transient immunosuppression [7]. The last phase of MDV infection is characterized by reactivation of the virus with the second cycle of lytic infection, induction of T cell lymphomas, and permanent immunosuppression [32]. The MDV-induced immunosuppression increases the susceptibility of infected birds to other diseases such as chicken anemia virus, reovirus, and infectious bursal disease virus [3]. It is believed that latently infected T cells migrate through the bloodstream and establish lymphomas in the visceral organs, peripheral nerves, and skin [7]. Fully infectious enveloped cell-free virions are produced in feather follicle epithelial cells (FFE) and eventually released into the environment with molted feathers and dander to infect contact birds [8].
MDV strains have been categorized into three serotypes based on antigenic differences and biological features. Oncogenic strains, including their attenuated forms, are classified as serotype 1, while non-oncogenic strains isolated from chickens and turkeys are classified as serotypes 2 and 3, respectively [1119]. The attenuated serotype 1 CVI988/Rispens and the non-oncogenic serotypes 2 and 3 (SB-1 and HTV, respectively) have been used as vaccines to prevent and control MD [262933]. The naturally attenuated serotype 1 CVI988/Rispens, which was first described by Rispens et al. [30], is a commercial vaccine and is considered the gold standard against highly pathogenic (very virulent and very virulent plus) MDV pathotypes [1229]. The attenuated serotype 1 viruses, used as vaccines, provide better protection and induce more effective immune responses, probably due to antigenic similarity with pathogenic strains of MDV [36]. The live MD vaccines prevent transient paralysis and lymphoma formation and mediate significant reductions in viral replication and assembly in FFE. They do not, however, prevent host infection, viral replication, and shedding [8].
Although MD vaccines have been in use for more than four decades, the specific mechanism of vaccine-induced protection is unclear. It has been shown that innate immune responses are induced but, unlike most other vaccines, adaptive immune system activation in response to MD vaccine is minimal [22]. Macrophages are essential cellular components of the innate immune defense system that have an essential role in shaping the adaptive immune system. Activated macrophages can greatly influence immune responses to viral infection and are able to spread through the production of several cytokines and chemokines including interleukin (IL)-1β, IL-6, IL-8, IL-12, interferons (IFNs), and tumor necrosis factor [128]. Studies have shown that macrophages play an important role in controlling initial MDV replication and the destruction of viral antigens [131439]. In vitro and in vivo studies have indicated that macrophages play a pivotal role in the pathogenesis of MD. Macrophages obtained from MDV-infected chickens can inhibit MDV replication in vitro much more efficiently than macrophages from control birds [16]. Macrophages from MD-susceptible line 72 have greater antiviral activities than macrophages of MD-resistant line 63 [27]. Although the mechanism of inhibition of MDV replication has not been fully described, it has been postulated that IFNs and macrophage activating factor may have essential roles [37]. While NO induces a direct inhibitory effect on viral replication and infection, other mediators released by activated macrophages regulate natural killer (NK) cell activation and IFN-γ production, leading to a positive feedback that increases the activation of both cell types within tissues and, ultimately, an effective innate immune response [5142532]. NK cell-derived IFN-γ inhibits viral replication, induces Th1-type immunity, and activates macrophages [1718].
In contrast to the amount of information on macrophage responses to MDV infection, there is a paucity of reports concerning the role of macrophages in MD vaccine-induced immunity. The purpose of the present study was to investigate the role of macrophages in CVI988/Rispens-mediated protection in MD-susceptible and MD-resistant chicken lines.
The specific-pathogen-free (SPF) chickens in the current study were from progenitor line 63 (MD-resistant) and 72 (MD-susceptible) birds [2]. These birds were from unvaccinated breeder hens and lacked antibodies to many avian pathogens including MDV, herpesvirus of turkey, reticuloendotheliosis virus and avian leucosis virus. The chicks were hatched and raised under SPF conditions at the Avian Disease and Oncology Laboratory (ADOL) poultry facility and housed in modified Horsfall-Bauer isolation units for the duration of the experiment. All animal experiments were approved and carried out in accordance with the guidelines set forth by the ADOL Institutional Animal Care and Use Committee (approval No. AADOL ACUC 15.15) and the Guidelines for Care and Use of Laboratory Animals published by Institute for Laboratory Animal Research in 1996.
One-day-old chicks from each line were randomly distributed into two groups of 15 birds each and kept in separate isolators. Birds from one group of each chicken line were vaccinated with CVI988/Rispens at post-hatch day 12, while the second group of each line served as the negative control. Three chickens from each group were euthanatized by CO2 inhalation and necropsied for tissue collection at 3, 5, and 10 days post-immunization (dpi). A portion of each duodenum and cecal tonsil (CT) was stored in RNAlater (Ambion, USA) to prevent RNA degradation. A small sample of each duodenum and CT was also put in optimal cutting temperature compound (Sakura Finetek USA, USA), snap-frozen in liquid nitrogen, and stored at −80℃ until used for immunohistochemical analysis. At 3 and 5 dpi, spleen tissues were aseptically removed and stored in Liebowitz-McCoy (LM) medium for macrophage isolation shortly after collection.
The CVI988/Rispens vaccine was purchased from Intervet (USA). Chickens were inoculated intraperitoneally with 2,000 plaque-forming unit (PFU) of the vaccine virus at day 12 post-hatch per manufacturer's instruction. One dose of vaccine contains approximately 2,000 PFU of the vaccine virus.
Pools of spleens from three vaccinated and three non-vaccinated chickens were decapsulated and gently passed through a size 60-mesh screen (Sigma-Aldrich, USA) to obtain a single-cell suspension. Splenocytes were re-suspended in cold LM medium (5% fetal bovine serum), harvested after centrifugation on a Histopaque-1077 (Sigma-Aldrich) at 700 × g for 30 min at room temperature to move dead cells and, then, washed twice in cold phosphate buffered saline (PBS). The pellet was re-suspended in cold degassed buffer (PBS, 0.5% bovine serum albumin, 2 mM EDTA, pH 7.2), and the number of cells was determined by using a TC-10 automated cell counter (Bio-Rad Laboratories, USA). Then, the cells were labeled with mouse monoclonal antibody KUL-01 (SouthernBiotech, USA) to identify chicken macrophages and monocytes [232435], according to the manufacturer's recommendations. MicroBeads coated with goat anti-mouse IgG1 (Miltenyi Biotec, USA) were used for separation of macrophages from the mixed splenocytes according to the manufacturer's recommendations. An LS column and a MACS Separator (Miltenyi Biotec) were used to collect macrophages according to the total number of live cells and the number of magnetically labeled cells. Macrophages were suspended with TRIzol (Invitrogen, USA) and stored at −80℃ for RNA isolation. To prevent unwanted activation of macrophages in vitro, spleen tissues were kept on ice and ice-cold solutions were used for separation of macrophages.
Total RNA was isolated from the homogenized duodenum and CT tissues or isolated spleen macrophages of three birds of each group (see Experiment design) at days 3, 5, and 10 dpi; (three biological replications) by using Tri Reagent RT (Molecular Research Center, USA) according to the manufacturer's instructions.
Real-time polymerase chain reaction (PCR) analysis for relative quantification of chicken gene expression transcripts was carried out at the Research Technology and Support Facility of Michigan State University in East Lansing, Michigan, USA. Each reaction mix (10 µL) was prepared by adding 5 µL SYBR Green PCR master mix (2×; Applied Biosystems, USA), 2.5 µL of 1:20 dilution of cDNA template (corresponding to 2 µg of the starting amount of RNA), and 2.5 µL each of the specific primers (25 µM). The amplification reactions were carried out at 50℃ for 2 min, 95℃ for 10 min, 40 cycles at 95℃ for 15 sec followed by 57℃ for 1 min. All reactions were run in three biologic replications in a 7900HT Sequence Detection System (Applied Biosystems). The primers for chicken genes were designed by using MacVector software (ver. 14.5.3; Accelrys, USA), and all the primers were synthesized by MWG Biotech (USA). The primers sequences and their amplicons are listed in Table 1 [40]. The housekeeping gene β-actin was included in the assay to ensure that similar amounts of cDNA were used in each reaction and that a uniform amplification process was obtained.
The frozen tissue samples, embedded in optimal cutting temperature, were sectioned at 5 microns, placed on 2% 3-aminopropyltriethoxysilane-coated slides, and air dried overnight. The microtome sections were fixed in formal acetate fixative at room temperature for 10 min and then rinsed three times in Tris-buffered saline for 5 min each. To block the endogenous peroxidase activity, the samples were treated with 0.3% hydrogen peroxide in Tris-buffered saline for 20 min followed by rinses with tap and distilled water. All of the avidin-biotin-complex staining steps were performed at room temperature by using a DAKO Autostainer (Agilent Technologies, USA) followed by rinses in Tris-buffered saline with Tween 20 (Scytek Laboratories, USA). Non-specific proteins were blocked with normal horse serum diluted in PBS (1/30 dilution; Vector Laboratories, USA) for 30 min. Sections were then incubated with an avidin-biotin blocking system for 15 min each (Avidin D [Vector Laboratories] and d-Biotin [Sigma-Aldrich]). For detection of specific immune cells, tissue samples were incubated with mouse anti-chicken macrophage or CD3+ T cell monoclonal antibodies at working dilutions of 1:200 (KUL01 and CT-3, respectively; SouthernBiotech) for 1 h in normal antibody diluent (NAD; Scytek Laboratories). Samples were rinsed and then incubated with biotinylated horse anti-mouse IgG (H + L) prepared at 11.5 µg/mL in NAD and incubated for 60 min. For detection of CVI988/Rispens antigen, mouse anti-gB monoclonal antibody developed at the ADOL poultry facility was used [19] at a 1:1,000 working dilution. Samples then were further treated with ready to use (R.T.U.) Vector Elite Peroxidase Reagent (Vector Laboratories) for 30 min. Finally, the tissues were incubated with Vector NovaRED peroxidase chromogen (Vector Laboratories) for reaction development for 15 min, followed by counterstaining in Gill hematoxylin (Thermo Fisher, USA) for 15 sec.
Relative quantification of chicken gene expression was determined using a 2−ΔΔCT method [21]. The level of expression of the target genes (IL-1β, IL-6, IL-8, IL-12, IFN-α, IFN-γ) in CT, duodenum, and spleen macrophages of age- and line-matched control birds were used as references or baselines for calculation of fold changes in gene expression in the CVI988/Rispens-vaccinated chickens of each line. The expression of each gene was normalized to the expression level of housekeeping gene β-actin.
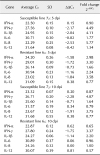
The expression pattern of selected immune-related genes associated with the activation of macrophages revealed no significant changes in the transcriptional activities of the tested genes in the duodenum of the vaccinated birds of either line at 3 dpi (data not shown). The expression levels of IL-1β, IL-8, and IFN-γ were upregulated in the duodenum of the vaccinated birds of MD-susceptible line 72 at 5 dpi (Table 2). In addition to IL-1β, IL-8, and IFN-γ, there was increase in the expression level of IFN-α in the duodenum of vaccinated birds of MD-resistant line 63 (Table 2). High transcriptional activity of IFN-γ was detected in the duodenum of birds of both vaccinated lines at 10 dpi (Table 2). IL-8 expression was extended to 10 dpi in the duodenum of vaccinated birds of susceptible line 72 (Table 2). In contrast to duodenum results, IL-8 and IFN-γ were both upregulated in the CT of both vaccinated lines at 3 dpi (Table 3). Additionally, compared to susceptible line 72, IL-1β showed higher transcriptional activity in the CT of vaccinated birds of resistant line 63 at 3 dpi (Table 3). Notably high expression of some of the tested genes was observed in the CT of vaccinated birds at 5 dpi (Table 3). The expression level of IFN-γ was almost 40-fold higher in the vaccinated birds of susceptible line 72 than in the unvaccinated control birds. IL-12 expression was over 26-fold higher in vaccinated susceptible line 72 birds than in the unvaccinated control birds (Table 3). The expression level of IFN-γ was 10-fold higher in the CT of vaccinated resistant line 63 birds than in the age-matched controls at the same sampling time (Table 3). Overexpression of IFN-γ and IL-12 was extended to 10 dpi in the CT of the vaccinated birds of susceptible line 72 (Table 3). Upregulation of IFN-γ was also detected in the CT of the vaccinated birds of resistant line 63 at 10 dpi (Table 3).
Gene expression analysis of isolated spleen macrophages revealed differential expression patterns between the vaccinated birds of the MD-resistant and MD-susceptible lines at 3 and 5 dpi. IFN-α was the only gene with higher expression level in the spleen macrophages of the susceptible line birds at 3 dpi (Table 4). No significant changes were observed in the expression levels of other tested genes in the spleen macrophages of vaccinated birds of susceptible line 72 at 3 or 5 dpi (Table 4). Substantially higher expression levels of IFN-γ (41-fold change), IL-6 (19-fold change), IL-8 (9-fold change), and IL-12 (166-fold change) were observed in the spleen macrophages of the vaccinated resistant line 63 birds at 3 dpi (Table 4). To a lesser extent, similar patterns of upregulation were detected in most of the tested genes in the resistant line 63 birds at 5 dpi (Table 4).
Although no specific software was used in determining accurate cell population estimates within the tested tissues, immunohistochemical analysis of CD3+ T cells revealed no visible population increase in the duodenum of the vaccinated birds at 5 dpi (panels B and D in Fig. 4; lines 63 and 72, respectively) when compared to the corresponding age-matched control birds (panels A and C in Fig. 4; lines 63 and 72, respectively). Macrophage populations, on the other hand, clearly increased in the duodenum of both the MD-resistant and MD-susceptible lines at 5 dpi (panels F and H in Fig. 4; lines 63 and 72, respectively) when compared to the non-vaccinated control birds (panels E and G in Fig. 4; lines 63 and 72, respectively).
We used anti-gB monoclonal (glycoprotein of CVI988) antibody to detect the presence of virus particles within the CT and duodenum of the vaccinated birds. No virus-specific antigen was detected in the duodenum or CT of the vaccinated birds of resistant line 63 at 5 dpi (panels B and D in Fig. 5, respectively). The corresponding control birds were also negative for the virus antigen (panels A and C in Fig. 5, respectively). However, there was a significant number of virus particles present in the duodenum and CT of the vaccinated birds of susceptible line 72 (panels F and H in Fig. 5, respectively). Panels E and G in Fig. 5 present the corresponding duodenum and CT tissue results, respectively, for the negative birds.
MDV, the etiologic agent of MD, is an alpha-herpesvirus that causes various clinical symptoms including anemia, weight loss, transient paralysis, bursal/thymic atrophy, T cell lymphomas, and immunosuppression [41015]. Although MD has been controlled by vaccination since the 1970s [6], the underlying mechanism behind its protection is unclear. Unlike most animal and human vaccines that require activation and direct involvement of adaptive immune system for proper protection, MD vaccines induce partial protection as early as 24 to 48 h post-vaccination [20]. Depending on the protective efficacy of the vaccine used, the immunity provided can attain 95% to 100% by day 5 post-vaccination [20].
The focus of this study was to investigate the effect of vaccination on the activation of macrophages within spleen, CT, and duodenum tissues of MD-susceptible and MD-resistant chicken lines by analyzing the expression patterns of selected immune-related genes associated with activation of macrophages. Additionally, we used immunohistochemical analysis to evaluate the populations of CD3+ T cells and macrophages in the tissues of control and vaccinated birds at day 5 post-vaccination. The results obtained from our gene expression analysis revealed no significant changes in the expression patterns of tested genes at 3 dpi in the duodenum of either MD line. At 5 dpi, however, IFN-γ, IL-1β, and IL-8 were upregulated in the duodenum of birds of MD-susceptible line 72. IFN-α, IFN-γ, IL-6, and IL-8 showed higher transcriptional activities in the duodenum of the vaccinated birds of MD-resistant line 63 when compared to the age-matched control birds (Table 2). At day 10 post-vaccination, the expressions of IFN-γ and IL-8 were higher in the duodenum of susceptible line 72 than in the age-matched control birds. IFN-γ was also upregulated in the duodenum of the vaccinated birds of resistant line 63. It is safe to assume that, at day 10 post-vaccination, the adaptive immune system is activated and, consequently, T and possibly B cells are contributing to the increased expression of some of the tested genes. It should also be pointed out that NK cells, being one of the major cellular components of the innate immune system, are likely involved in vaccine-mediated protection [34]. Killing of virus-infected and tumor cells in a non-major histocompatibility complex-restricted manner is mediated by exocytosis of granules and release of perforin and granzymes and production of IFN-γ, which leads to apoptosis of target cells and has a direct effect on the outcome of adaptive immune responses [59313841]. Therefore, it is more than likely that NK cells also contribute to the upregulation of IFN-γ in the tested tissues of the vaccinated birds.
Unlike duodenum, upregulation of IFN-γ and IL-8 was detected in the CT of both chicken lines at 3 dpi. In addition, IL-1β showed high transcriptional activity in the CT of resistant line 63 birds (Table 3). The highest levels of expression of the tested genes were in the CT of the vaccinated birds at day 5 post-vaccination. IFN-γ and IL-12 were significantly upregulated in the CT of the vaccinated birds of susceptible line 72 when compared to the unvaccinated control birds (39- and 26-fold increases, respectively). The expression level of IFN-γ in the CT of resistant line 63 birds was also considerably higher than that of the control birds (10-fold increase; Table 3). Once again, the high expressions of some of the tested genes were extended to day 10 post-vaccination, which could be partially due to the activation and contribution of the adaptive immune system (Table 3).
IFN-α was the only gene with a higher expression level in the isolated spleen macrophages of vaccinated susceptible line 72 birds than that of resistant line 63 birds at day 3 post-vaccination (9-fold increase). In contrast, IFN-γ (41-fold increase), IL-6 (19-fold increase), IL-8 (9-fold increase), and IL-12 (166-fold increase) exhibited the highest levels of expression in the spleen macrophages of resistant line 63 birds at day 3 post-vaccination. Similar patterns of expression, but with smaller fold changes, were observed for IFN-γ, IL-1β, IL-6 and IL-12 in the spleen macrophages of the vaccinated birds of resistant line 63 at 5 dpi (Table 4). The overall gene expression pattern in the tested tissues of both chicken lines are depicted in Figs. 1, 2, 3 as bar graphs for improved visualization (duodenum, CT, and spleen macrophages, respectively).
To test the possibility of activation and contribution of the adaptive immune system in the upregulation of tested genes, we undertook immunohistochemical analysis to evaluate the population of CD3+ T cells in the duodenum of control and vaccinated birds of both MD lines at 5 dpi. The results revealed no detectable increases in the populations of T cells within the duodenum of the vaccinated birds of either line when compared to the age- and line-matched control birds (panels A–D in Fig. 4). Immunohistochemical analysis of macrophages, however, showed a significant increase in the population of the phagocytic cells within the duodenum of vaccinated birds of both lines at 5 dpi (panels E–H in Fig. 4). There was insufficient CT tissue available to test the populations of CD3+ T cells or macrophages at either 3 or 5 dpi.
Immunohistochemical analysis detected no CVI988/Rispins antigen within the CT or duodenum of the vaccinated birds of the resistant line at 5 dpi. A substantial number of virus particles, however, were detected within the CT and duodenum of the MD-susceptible line (Fig. 5). It is possible that the inhibitory effect of the vaccine virus on macrophage function in the susceptible line 72 birds resulted in a lack of activation of NK cells and elimination of virus particles. The absence of any detectable CVI988/Rispens antigens within the tested tissues of the resistant line 63 birds is probably an indication that the virus was unable to break the macrophage barrier, resulting in activation of NK cells and, ultimately, clearance of the viral infection.
Macrophages and NK cells of the innate immune system play critical roles in vaccine-mediated protection against pathogenic strains of MDV. This study sheds light on the possible role of the innate immune system in vaccine-mediated protection and points the way for future development of a recombinant vaccine that specifically modulates innate immune responses.
Figures and Tables
Fig. 1
Bar graphs showing gene expression patterns within the duodenum of both Marek's disease chicken lines at 5 and 10 days post-immunization (dpi). For a statistical analysis summary, please see Table 2. IFN, interferon; IL, interleukin.

Fig. 2
Bar graphs showing the expression patterns of the tested genes within the cecal tonsils of both Marek's disease lines at 3, 5, and 10 days post-immunization (dpi). For a statistical analysis summary, please see Table 3. IFN, interferon; IL, interleukin.

Fig. 3
Bar graphs showing the expression patterns of the tested genes in spleen macrophages of both Marek's disease lines at 3 and 5 days post-immunization (dpi). For a statistical analysis summary, please see Table 4. IFN, interferon; IL, interleukin.

Fig. 4
Images from the immunohistochemical analysis of CD3+ T cells and macrophage populations within the duodenum of control and vaccinated birds of the resistant and susceptible Marek's disease lines at 5 days post-immunization. The tissue samples were incubated with combination of mouse anti-chicken CD3+ T cells or macrophage monoclonal antibodies (primary) and biotinylated horse anti-mouse IgG (secondary) for detection of specific immune cells. Panels B and D show the CD3+ T cell populations within the duodenum of vaccinated birds of the resistant and susceptible lines, respectively, while panels A and C present the corresponding control tissues. Panels F and H show the macrophage populations within the duodenum of vaccinated birds of the resistant and susceptible lines, respectively, while panels E and G show the corresponding non-vaccinated control tissues. Scale bars = 200 µm (A–D), 20 µm (E–H).
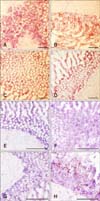
Fig. 5
Images from the immunohistochemical analysis of CVI988/Rispens antigen gB within the duodenum and the cecal tonsil (CT) of the vaccinated birds of the resistant and susceptible lines at 5 days post-immunization. The tissue samples were incubated with combination of mouse anti-gB monoclonal antibody (primary) and biotinylated horse anti-mouse IgG for detection CVI988 virions. Panels B and D show the duodenum and CT tissues of birds of vaccinated resistant line 63, respectively, stained with the anti-gB of CVI988/Rispens, while panels A and C, respectively, are the corresponding non-vaccinated control tissues. The gB antigen of CVI988/Rispens is depicted within duodenum and the CT of the vaccinated birds of the susceptible line 72 in panels F and H, respectively, while the corresponding control tissues are represented in panels E and G, respectively. Scale bars = 200 µm (A–H).
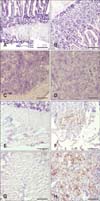
Table 2
Relative fold differences in chicken gene expression in duodenum at 5 and 10 dpi obtained by using the comparative CT method
Acknowledgments
The authors would like to thank Jennifer Pierluissi for excellent technical assistance.
References
1. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014; 5:491.

2. Bacon LD, Hunt HD, Cheng HH. A review of the development of chicken lines to resolve genes determining resistance to diseases. Poult Sci. 2000; 79:1082–1093.

3. Baigent S, Davison T. Marek's disease virus: biology and life cycle. In : Davison F, Nair V, editors. Marek's Disease: An Evolving Problem. Oxford: Elsevier Academic Press;2004. p. 62–77.
4. Biggs PM. The Leeuwenhoek Lecture, 1997. Marek's disease herpesvirus: oncogenesis and prevention. Philos Trans R Soc Lond B Biol Sci. 1997; 352:1951–1962.

5. Biron CA. Initial and innate responses to viral infections-pattern setting in immunity or disease. Curr Opin Microbiol. 1999; 2:374–381.
6. Blaxland JD, Macleod AJ, Hall T. Trials with Marek's disease vaccines prepared from a turkey herpes virus and an attenuated Marek's disease virus. Vet Rec. 1975; 97:50–52.

7. Calnek BW. Pathogenesis of Marek's disease virus infection. Curr Top Microbiol Immunol. 2001; 255:25–55.

8. Calnek BW, Adldinger HK, Kahn DE. Feather follicle epithelium: a source of enveloped and infectious cell-free herpesvirus from Marek's disease. Avian Dis. 1970; 14:219–233.

11. Davison F, Nair V. Marek's Disease: An Evolving Problem. The Netherlands and Boston: Elsevier Press;2004.
12. de Boer GF, Groenendal JE, Boerrigter HM, Kok GL, Pol JM. Protective efficacy of Marek's disease virus (MDV) CVI-988 CEF65 clone C against challenge infection with three very virulent MDV strains. Avian Dis. 1986; 30:276–283.

13. Djeraba A, Bernardet N, Dambrine G, Quéré P. Nitric oxide inhibits Marek's disease virus replication but is not the single decisive factor in interferon-gamma-mediated viral inhibition. Virology. 2000; 277:58–65.

14. Haffer K, Sevoian M, Wilder M. The role of the macrophages in Marek's disease: in vitro and in vivo studies. Int J Cancer. 1979; 23:648–656.

15. Jarosinski KW, Tischer BK, Trapp S, Osterrieder N. Marek's disease virus: lytic replication, oncogenesis and control. Expert Rev Vaccines. 2006; 5:761–772.

16. Kodama H, Mikami T, Inoue M, Izawa H. Inhibitory effects of macrophages against Marek's disease virus plaque formation in chicken kidney cell cultures. J Natl Cancer Inst. 1979; 63:1267–1271.
17. Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008; 8:259–268.

18. Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008; 9:495–502.

19. Lee LF, Liu X, Witter RL. Monoclonal antibodies with specificity for three different serotypes of Marek's disease viruses in chickens. J Immunol. 1983; 130:1003–1006.
20. Lee LF, Zhang H, Heidari M, Lupiani B, Reddy SM. Evaluation of factors affecting vaccine efficacy of recombinant Marek's disease virus lacking the Meq oncogene in chickens. Avian Dis. 2011; 55:172–179.

21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001; 25:402–408.

22. Markowski-Grimsrud CJ, Schat KA. Cytotoxic T lymphocyte responses to Marek's disease herpesvirus-encoded glycoproteins. Vet Immunol Immunopathol. 2002; 90:133–144.

23. Mast J, Goddeeris BM. Development of immunocompetence of broiler chickens. Vet Immunol Immunopathol. 1999; 70:245–256.

24. Mast J, Goddeeris BM, Peeters K, Vandesande F, Berghman LR. Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01. Vet Immunol Immunopathol. 1998; 61:343–357.

25. Matikainen S, Paananen A, Miettinen M, Kurimoto M, Timonen T, Julkunen I, Sareneva T. IFN-α and IL-18 synergistically enhance IFN-γ production in human NK cells: differential regulation of Stat4 activation and IFN-γ gene expression by IFN-α and IL-12. Eur J Immunol. 2001; 31:2236–2245.

26. Okazaki W, Purchase HG, Burmester BR. Protection against Marek's disease by vaccination with a herpesvirus of turkeys. Avian Dis. 1970; 14:413–429.

27. Powell PC, Hartley KJ, Mustill BM, Rennie M. Studies on the role of macrophages in Marek's disease of the chicken. J Reticuloendothel Soc. 1983; 34:289–297.
29. Rispens BH, van Vloten H, Mastenbroek N, Maas HJ, Schat KA. Control of Marek's disease in the Netherlands. I. Isolation of an avirulent Marek's disease virus (strain CVI 988) and its use in laboratory vaccination trials. Avian Dis. 1972; 16:108–125.

30. Rispens BH, van Vloten H, Mastenbroek N, Maas JL, Schat KA. Control of Marek's disease in the Netherlands. II. Field trials on vaccination with an avirulent strain (CVI 988) of Marek's disease virus. Avian Dis. 1972; 16:126–138.

32. Schat K, Nair V. Marek's disease. In : Saif YM, Fadly AM, Glisson JR, McDougald LB, Nolan LK, Swayne DE, editors. Diseases of Poultry. Ames: Iowa State University Press;2008. p. 452–514.
33. Schat KA, Calnek BW. Characterization of an apparently nononcogenic Marek's disease virus. J Natl Cancer Inst. 1978; 60:1075–1082.
34. Sharma JM. Natural killer cell activity in chickens exposed to Marek's disease virus: inhibition of activity in susceptible chickens and enhancement of activity in resistant and vaccinated chickens. Avian Dis. 1981; 25:882–893.

35. Van Immerseel F, De Buck J, De Smet I, Mast J, Haesebrouck F, Ducatelle R. The effect of vaccination with a Salmonella Enteritidis aroA mutant on early cellular responses in caecal lamina propria of newly-hatched chickens. Vaccine. 2002; 20:3034–3041.

36. Venugopal K. Marek's disease: an update on oncogenic mechanisms and control. Res Vet Sci. 2000; 69:17–23.

37. VonBülow V, Weiler H, Klasen A. Activating effects of interferons, lymphokines and viruses on cultured chicken macrophages. Avian Pathol. 1984; 13:621–637.

38. White DW, Keppel CR, Schneider SE, Reese TA, Coder J, Payton JE, Ley TJ, Virgin HW, Fehniger TA. Latent herpesvirus infection arms NK cells. Blood. 2010; 115:4377–4383.

39. Xing Z, Schat KA. Inhibitory effects of nitric oxide and gamma interferon on in vitro and in vivo replication of Marek's disease virus. J Virol. 2000; 74:3605–3612.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download