Abstract
Objective
Methods
Results
REFERENCES
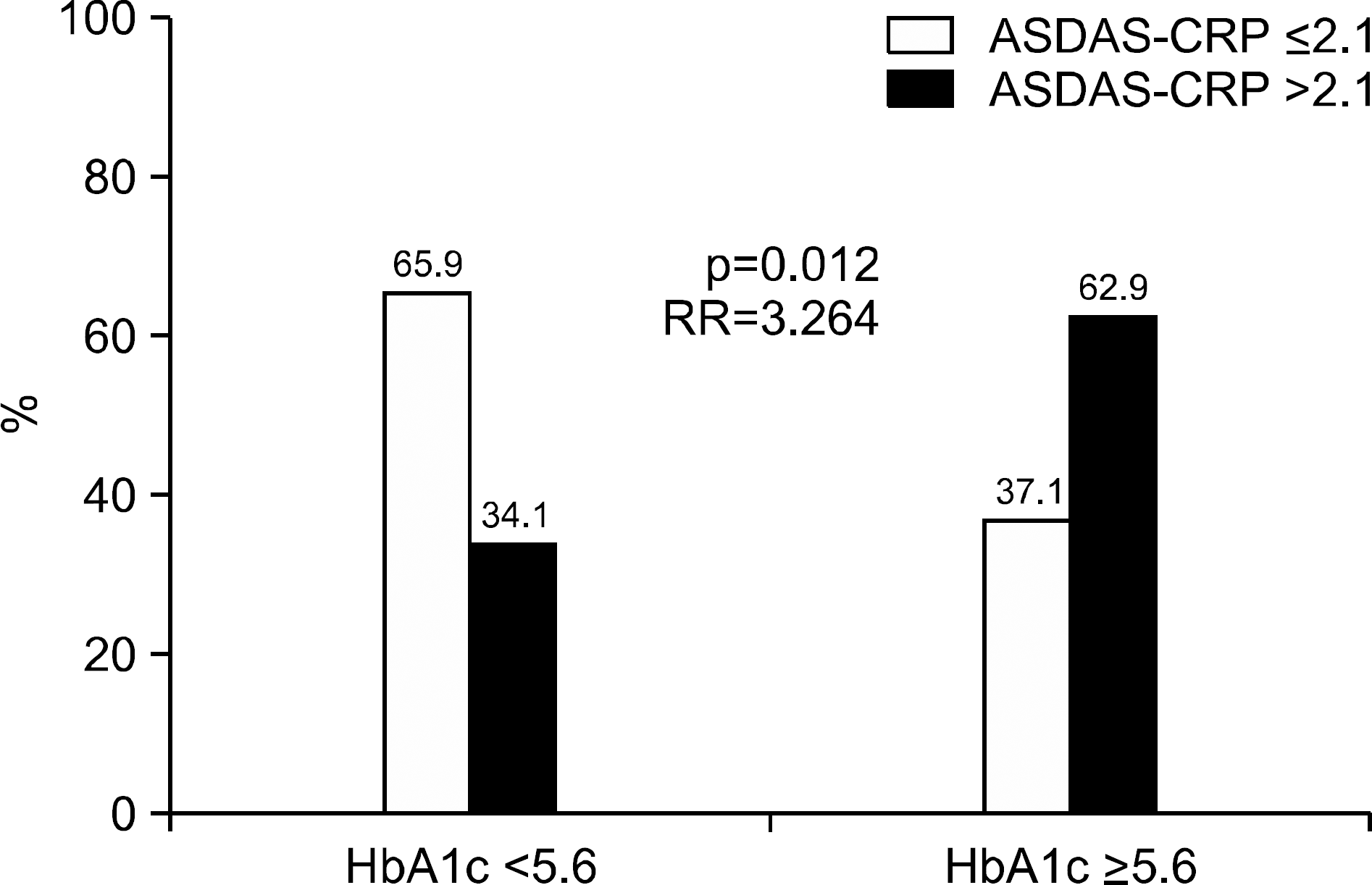 | Figure 1.Optimal cutoff values of HbA1c to reflect active ankylosing spondylitis. Active ankylosing spondylitis in patients having HbA1c ≥5.6 was identified more often than in those having HbA1c <5.6 (62.9% vs. 34.1%, p=0.012). Patients having HbA1c more than 5.6 showed significantly enhanced risk of active AS than those having not (RR=3.264). ASDAS: ankylosing spondylitis disease activity score, CRP: C-reactive protein, RR: relative risk, HbA1c: hemoglobin A1c, AS: ankylosing spondylitis. |
Supplementary Table 1.
| BMI | HbA1c | GA | Fasting glucose | ASDAS-ESR | ASDAS-CRP | BASDAI | BAS-G | |
|---|---|---|---|---|---|---|---|---|
| BMI | 1 | |||||||
| HbA1c | 0.227* | 1 | ||||||
| GA | −0.268* | 0.400* | 1 | |||||
| Fasting glucose | 0.331* | 0.405* | 0.186 | 1 | ||||
| ASDAS-ESR | 0.013 | 0.220 | 0.105 | 0.011 | 1 | |||
| ASDAS-CRP | 0.032 | 0.315* | 0.135 | 0.037 | 0.792* | 1 | ||
| BASDAI | 0.019 | 0.226* | 0.192 | 0.010 | 0.719* | 0.774* | 1 | |
| BAS-G | 0.039 | 0.401* | 0.221 | 0.166 | 0.568* | 0.617* | 0.655* | 1 |
| BASFI | −0.118 | 0.124 | 0.111 | 0.013 | 0.376* | 0.322* | 0.211 | 0.279* |
BMI: body mass index, HbA1c: haemoglobin A1c, GA: glycated albumin, ASDAS: ankylosing spondylitis disease activity score, BASDAI: Bath ankylosing spondylitis disease activity index, BAS-G: Bath ankylosing spondylitis patient global score, BASFI: Bath ankylosing spondylitis disease activity functional index.
Table 1.
Values are expressed as median (interquartile range, IQR) or number (%). BMI: body mass index, HLA: human leukocyte antigen, HbA1c: hemoglobin A1c, GA: glycated albumin, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, ASDAS: ankylosing spondylitis disease activity score, BASDAI: bath ankylosing spondylitis disease activity index, BAS-G: bath ankylosing spondylitis patient global score, BASFI: bath ankylosing spondylitis disease activity functional index, TNF: tumour necrosis factor.
Table 2.
ASDAS: ankylosing spondylitis disease activity score, ESR: erythrocyte sedimentation rate, BMI: body mass index, HbA1c: hemoglobin A1c, GA: glycated albumin, CRP: C-reactive protein, TNF: tumour necrosis factor, N/A: not available. *CRP was not included in multivariate analysis, because CRP is a variable closely correlated with ESR (ASDAS-ESR) in inflammation in order not to confound the interpretation of statistical results.
Table 3.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Regression coefficient (crude B) | Correlation coefficient (R=β) | p-value | Standardized β* | 95% confidential interval | p-value | |
| Demographic data | ||||||
| Age (yr) | 0.013 | 0.164 | 0.158 | |||
| Follow-up duration (yr) | 0.015 | 0.105 | 0.367 | |||
| BMI (kg/m2) | 0.008 | 0.032 | 0.781 | |||
| Laboratory results | ||||||
| HbA1c (%) | 0.972 | 0.315 | 0.006 | 0.234 | 0.060, 1.383 | 0.033 |
| GA (%) | 0.091 | 0.135 | 0.244 | |||
| Fasting glucose (mg/dL) | 0.004 | 0.037 | 0.750 | |||
| ESR (mm/h) | 0.026 | 0.477 | <0.001 | |||
| CRP (mg/L) | N/A | N/A | N/A | |||
| Ferritin (mg/dL) | −0.003 | −0.199 | 0.150 | |||
| White blood cell (/mm3) | 0.133 | 0.288 | 0.012 | 0.265 | 0.022, 0.222 | 0.017 |
| Hemoglobin (g/dL) | −0.137 | −0.241 | 0.036 | −0.204 | −0.248, 0.016 | 0.084 |
| Platelet×103 (/mm3) | 0.002 | 0153 | 0.186 | |||
| Serum albumin (mg/dL) | −0.811 | −0.262 | 0.022 | −0.099 | −1.030, 0.419 | 0.404 |
| Blood urea nitrogen (mg/dL) | 0.027 | 0.104 | 0.373 | |||
| Creatinine (mg/dL) | −0.243 | −0.040 | 0.734 | |||
| Alkaline phosphatase (IU/L) | 0.011 | 0.212 | 0.068 | |||
| Aspartate aminotransferase (IU/L | L) 0.015 | 0.098 | 0.402 | |||
| Alanine aminotransferase (IU/L) | 0.014 | 0.145 | 0.213 | |||
| Total cholesterol (mg/dL) | 0.004 | 0.119 | 0.307 | |||
| High density cholesterol (mg/dL) | −0.012 | −0.159 | 0.246 | |||
| Low density cholesterol (mg/dL) | 0.002 | 0.069 | 0.609 | |||
| Triglyceride (mg/dL) | 0.004 | 0.184 | 0.166 | |||
| Medications | ||||||
| Glucocorticoid use | 0.147 | 0.044 | 0.707 | |||
| Methotrexate use | 0.236 | 0.060 | 0.604 | |||
| Sulfasalazine use Anti-TNF agents use | 0.349−0.325 | 0.180 −0.161 | 0.120 0.166 | |||
Table 4.
Values are expressed as mean±standard deviation or number (%). ASDAS: ankylosing spondylitis disease activity score, CRP: C-reactive protein, AS: ankylosing spondylitis, BMI: body mass index, HLA: human leukocyte antigen, HbA1c: hemoglobin A1c, GA: glycated albumin, ESR: erythrocyte sedimentation rate, BASDAI: bath ankylosing spondylitis disease activity index, BAS-G: bath ankylosing spondylitis patient global score, BASFI: bath ankylosing spondylitis disease activity functional index, TNF: tumour necrosis factor, N/A: not available.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download