Abstract
Objective
The choice between primary debulking surgery (PDS) and neoadjuvant chemotherapy (NAC) in advanced ovarian cancer remains controversial. We evaluated NAC use in our center before and after results from a randomized trial were published, with the aim to determine the impact of changes in the neoadjuvant strategy on survival in advanced-stage ovarian cancer.
Methods
We retrospectively investigated the clinical course of 435 patients with ovarian, tubal, or peritoneal carcinoma (International Federation of Gynecology and Obstetrics [FIGO] stage III or IV). According to the period of treatment, we stratified patients into a control group (n=216; diagnosed between 2006 and 2010; 83.8% underwent PDS) and a study group (n=219; diagnosed between 2011 and 2014; 48.9% received NAC followed by interval debulking surgery [IDS]).
Results
There were no between-group differences in age, body mass index, histology findings, or tumor grade. Compared to patients in the control group, those in the study group were more likely to receive NAC followed by IDS as first-line treatment (48.9% vs. 16.2%; p<0.001), cytoreductive surgery to no-residual disease (21.5% vs. 10.2%; p<0.001), or radical surgery (57.5% vs. 35.6%; p<0.001). However, there was no between-group difference in postoperative morbidity. Kaplan-Meier analysis showed no between-group differences in progression-free or overall survival (p=0.449 and 0.952, respectively).
Go to : 
References
1. Berkenblit A, Cannistra SA. Advances in the management of epithelial ovarian cancer. J Reprod Med. 2005; 50:426–38.
2. Lee JY, Kim EY, Jung KW, Shin A, Chan KK, Aoki D, et al. Trends in gynecologic cancer mortality in East Asian regions. J Gynecol Oncol. 2014; 25:174–82.

3. Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999–2010. J Gynecol Oncol. 2013; 24:298–302.

4. Morgan RJ Jr, Alvarez RD, Armstrong DK, Burger RA, Castells M, Chen LM, et al. Ovarian cancer, version 3.2012. J Natl Compr Canc Netw. 2012; 10:1339–49.

5. Onda T, Matsumoto K, Shibata T, Sato A, Fukuda H, Konishi I, et al. Phase III trial of upfront debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0602. Jpn J Clin Oncol. 2008; 38:74–7.

6. Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010; 363:943–53.

7. Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015; 386:249–57.

8. Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): final analysis of perioperative outcome. Eur J Cancer. 2016; 59:22–33.

9. Schott AF, Hayes DF. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2012; 30:1747–9.

10. Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006; 103:1070–6.

11. Kang S, Nam BH. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Ann Surg Oncol. 2009; 16:2315–20.

12. Fagotti A, Vizzielli G, Fanfani F, Costantini B, Ferrandina G, Gallotta V, et al. Introduction of staging laparoscopy in the management of advanced epithelial ovarian, tubal and peritoneal cancer: impact on prognosis in a single institution experience. Gynecol Oncol. 2013; 131:341–6.

13. Fagotti A, Ferrandina G, Fanfani F, Garganese G, Vizzielli G, Carone V, et al. Prospective validation of a laparoscopic predictive model for optimal cytoreduction in advanced ovarian carcinoma. Am J Obstet Gynecol. 2008; 199:642.e1–6.

14. Eisenhauer EL, Abu-Rustum NR, Sonoda Y, Levine DA, Poynor EA, Aghajanian C, et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol Oncol. 2006; 103:1083–90.

15. Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009; 114:26–31.

16. Wimberger P, Lehmann N, Kimmig R, Burges A, Meier W, Du Bois A. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR). Gynecol Oncol. 2007; 106:69–74.

17. Aletti GD, Eisenhauer EL, Santillan A, Axtell A, Aletti G, Holschneider C, et al. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol Oncol. 2011; 120:23–8.

18. Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol. 2004; 94:650–4.

19. Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006; 107:77–85.

20. Ren Y, Jiang R, Yin S, You C, Liu D, Cheng X, et al. Radical surgery versus standard surgery for primary cytoreduction of bulky stage IIIC and IV ovarian cancer: an observational study. BMC Cancer. 2015; 15:583.

21. Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996; 335:1950–5.

22. Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006; 354:34–43.

23. Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001; 19:1001–7.

Go to : 
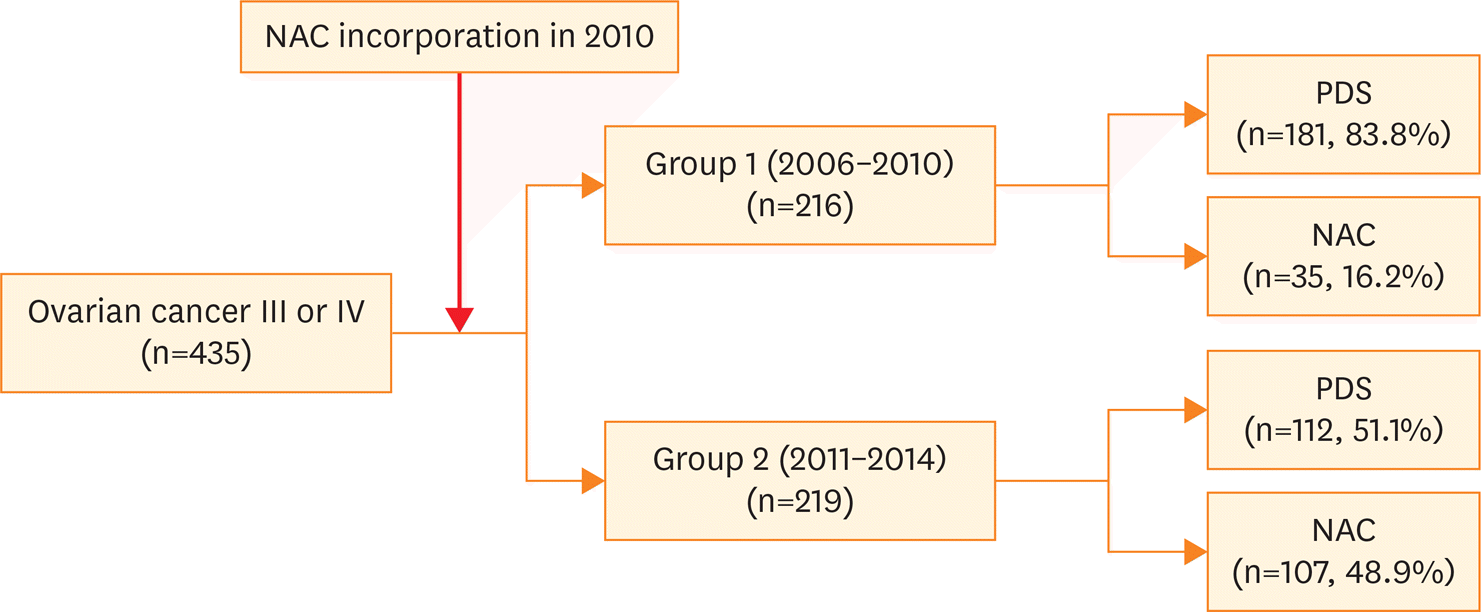 | Fig. 1.Change in NAC use between 2006 and 2014. NAC, neoadjuvant chemotherapy; PDS, primary debulking surgery. |
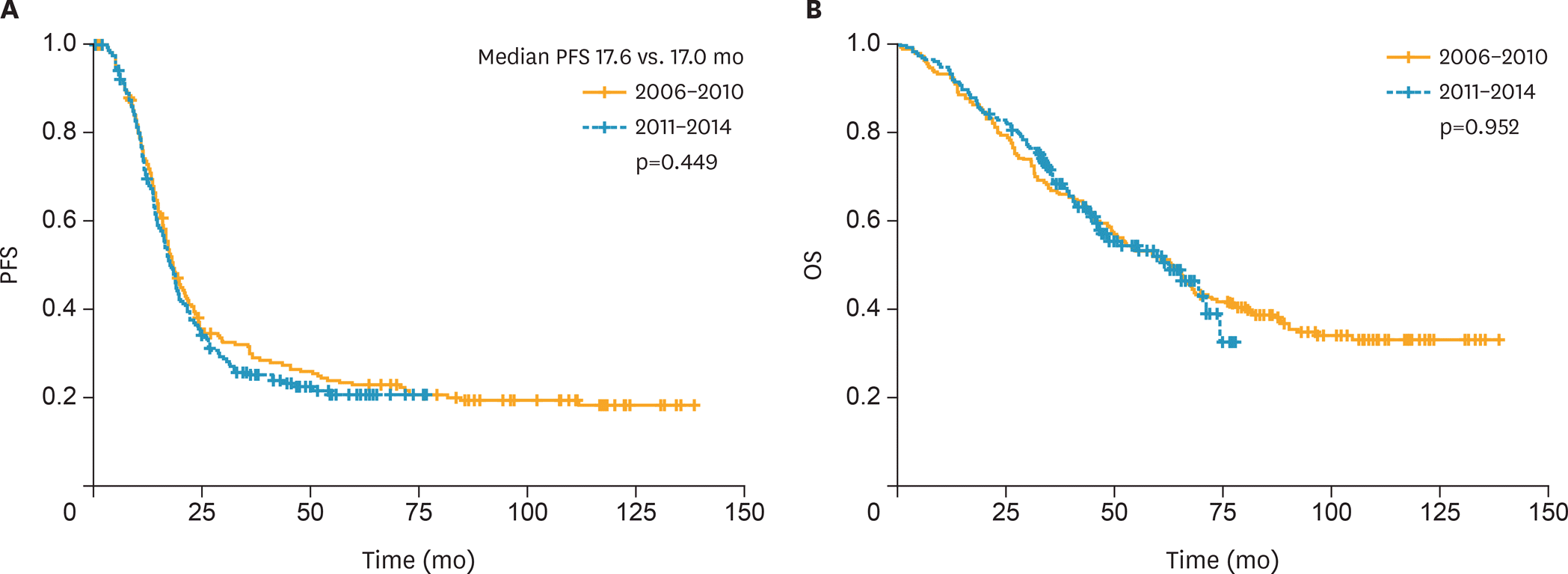 | Fig. 2.Kaplan-Meier curves of PFS (A) and OS (B) according to time period (2006–2010 vs. 2011–2014). OS, overall survival; PFS, progression-free survival. |
Table 1.
Patient and clinical characteristics
Table 2.
Classification of postoperative outcomes according to the Memorial Sloan-Kettering Cancer Center's surgical secondary events grading system
| Variables | Group 1 (n=216) | Group 2 (n=219) | p |
|---|---|---|---|
| Complication grade* | 0.068 | ||
| 0 | 102 (47.2) | 130 (59.4) | |
| 1 | 15 (6.9) | 9 (4.1) | |
| 2 | 79 (36.6) | 62 (28.4) | |
| 3 | 10 (4.7) | 10 (4.6) | |
| 4 | 0 (0) | 3 (1.4) | |
| 5 | 2 (3.7) | 1 (0.5) | |
| Not available | 8 (3.7) | 4 (1.8) | |
| Major complications | 0.465 | ||
| 0–2 | 196 (90.7) | 201 (91.8) | |
| 3–5 | 12 (5.6) | 14 (6.4) | |
| Not available | 8 (3.7) | 4 (1.8) | |
| Residual disease | <0.001 | ||
| No gross | 22 (10.2) | 47 (21.5) | |
| ≤1.0 cm | 94 (43.5) | 111 (50.7) | |
| >1.0 cm | 52 (24.1) | 13 (5.9) | |
| Not available | 48 (22.2) | 48 (21.9) | |
| PDS | <0.001 | ||
| No gross | 16 (8.8) | 17 (15.2) | |
| ≤1.0 cm | 77 (42.5) | 60 (53.6) | |
| >1.0 cm | 49 (27.1) | 8 (7.1) | |
| Not available | 39 (21.5) | 27 (24.1) | |
| NAC | 0.466 | ||
| No gross | 6 (17.1) | 30 (28.0) | |
| ≤1.0 cm | 17 (48.6) | 51 (47.7) | |
| >1.0 cm | 3 (8.6) | 5 (4.7) | |
| Not available | 9 (25.7) | 21 (19.6) | |
| Radical surgery† | <0.001 | ||
| None | 139 (64.4) | 93 (42.5) | |
| Any radical surgery | 77 (35.6) | 126 (57.5) | |
| Surgical complexity score groups‡ | 0.002 | ||
| 1 | 1 (0.5) | 0 (0) | |
| 2 | 207 (95.8) | 191 (87.2) | |
| 3 | 8 (3.7) | 28 (12.8) |
* According to the Memorial Sloan-Kettering Cancer Center's surgical secondary events grading system [16];




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download