Abstract
Objective
Locally advanced endometrioid adenocarcinoma (LA-EAC) accounts for the majority of deaths for this cancer, yet there is no consensus on adjuvant treatment after surgery. Past studies suggest that combined modality treatment (CMT) may improve outcomes over treatment with chemotherapy (CT) or radiation therapy (RT, either external beam radiotherapy [EBRT] or vaginal brachytherapy [VBT]) alone. Using a large US-based population-based registry, we evaluated adjuvant CMT in LA-EAC and the relative benefit of regional EBRT compared to focused VBT.
Methods
We studied patients diagnosed with Stage III LA-EAC between 2004 and 2013 from the National Cancer Data Base (NCDB). We used Cox regression and a log-rank test to assess survival based on treatment with CT alone, EBRT alone, VBT alone, or CMT with EBRT and/or VBT. We used a χ2 test to compare covariates between patients receiving CMT with EBRT or VBT.
Results
Patients who received CMT had better survival than those who received CT or EBRT/VBT alone. Compared to CMT with VBT, patients who received CMT with EBRT were slightly older and had more advanced-stage or positive nodes, and fewer had lymph node surgery. We found no survival difference between CMT with EBRT and CMT with VBT even when categorizing patients as high or low risk according to age, grade, and stage (low-risk p=0.3460; high-risk p=0.2158).
Go to : 
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.

2. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. 2016; 25:16–27.

4. Dowdy SC. Improving oncologic outcomes for women with endometrial cancer: realigning our sights. Gynecol Oncol. 2014; 133:370–4.

5. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016; 387:1094–108.

6. Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006; 24:36–44.

7. Homesley HD, Filiaci V, Gibbons SK, Long HJ, Cella D, Spirtos NM, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Gynecol Oncol. 2009; 112:543–52.

8. Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 2006; 103:155–9.

10. Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006; 95:266–71.

11. Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008; 108:226–33.

12. Wang CJ, Christie A, Folkert MR, Xie XJ, Albuquerque K. Temporal trends for adjuvant radiation utilization and their effect on survival for locally advanced (stage III) endometrial cancer in the USA. J Radiat Oncol. 2017; 6:175–87.

13. Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer–results from two randomised studies. Eur J Cancer. 2010; 46:2422–31.

14. Boothe D, Orton A, Odei B, Stoddard G, Suneja G, Poppe MM, et al. Chemoradiation versus chemotherapy or radiation alone in stage III endometrial cancer: patterns of care and impact on overall survival. Gynecol Oncol. 2016; 141:421–7.

15. Rauh-Hain JA, Pepin KJ, Meyer LA, Clemmer JT, Lu KH, Rice LW, et al. Management for elderly women with advanced-stage, high-grade endometrial cancer. Obstet Gynecol. 2015; 126:1198–206.

16. Klopp AH, Jhingran A, Ramondetta L, Lu K, Gershenson DM, Eifel PJ. Node-positive adenocarcinoma of the endometrium: outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol. 2009; 115:6–11.

17. Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006; 15:547–69.

18. Mundt AJ, McBride R, Rotmensch J, Waggoner SE, Yamada SD, Connell PP. Significant pelvic recurrence in high-risk pathologic stage I–IV endometrial carcinoma patients after adjuvant chemotherapy alone: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2001; 50:1145–53.

19. Secord AA, Geller MA, Broadwater G, Holloway R, Shuler K, Dao NY, et al. A multicenter evaluation of adjuvant therapy in women with optimally resected stage IIIC endometrial cancer. Gynecol Oncol. 2013; 128:65–70.

20. Milgrom SA, Kollmeier MA, Abu-Rustum NR, Tew WP, Sonoda Y, Barakat RR, et al. Postoperative external beam radiation therapy and concurrent cisplatin followed by carboplatin/paclitaxel for stage III (FIGO 2009) endometrial cancer. Gynecol Oncol. 2013; 130:436–40.

21. Kuku S, Williams M, McCormack M. Adjuvant therapy in stage III endometrial cancer: treatment outcomes and survival. A single-institution retrospective study. Int J Gynecol Cancer. 2013; 23:1056–64.
22. Albuquerque K, Folkert M, Mayadev J, Christie A, Liotta MR, Nagel C, et al. Adjuvant external radiation impacts outcome of pelvis-limited stage III endometrial carcinoma: a multi-institutional study. Am J Clin Oncol. Forthcoming. 2017.
23. Matei D, Filiaci VL, Randall M, Steinhoff M, DiSilvestro P, Moxley KM, et al. A randomized phase III trial of cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel vs. carboplatin and paclitaxel for optimally debulked, advanced endometrial carcinoma. J Clin Oncol. 2017; 35(suppl):abstr 5505.

24. De Boer SM, Powell ME, Mileshkin LR, Katsaros D, Bessette P, Haie-Meder C, et al. Final results of the international randomized PORTEC-3 trial of adjuvant chemotherapy and radiation therapy (RT) versus RT alone for women with high-risk endometrial cancer. J Clin Oncol. 2017; 35(suppl):abstr 5502.

25. Nout RA, Smit VT, Putter H, Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010; 375:816–23.

26. Huddleston A, Zhen S, Qi L, Rash D, Leiserowitz G, Mayadev J. The impact of a vaginal brachytherapy boost to pelvic radiation in stage III endometrial cancer. J Contemp Brachytherapy. 2015; 7:122–7.

27. Bingham B, Orton A, Boothe D, Stoddard G, Huang YJ, Gaffney DK, et al. Brachytherapy improves survival in stage III endometrial cancer with cervical involvement. Int J Radiat Oncol Biol Phys. 2017; 97:1040–50.

28. Modh A, Ghanem AI, Burmeister C, Rasool N, Elshaikh MA. Trends in the utilization of adjuvant vaginal brachytherapy in women with early-stage endometrial carcinoma: results of an updated period analysis of SEER data. Brachytherapy. 2016; 15:554–61.

29. Young MR, Higgins SA, Ratner E, Yu JB, Mani S, Silasi DA, et al. Adjuvant carboplatin, paclitaxel, and vaginal cuff brachytherapy for stage III endometrial cancer: analysis of outcomes and patterns of recurrence based on pathologic characteristics. Int J Gynecol Cancer. 2015; 25:431–9.
Go to : 
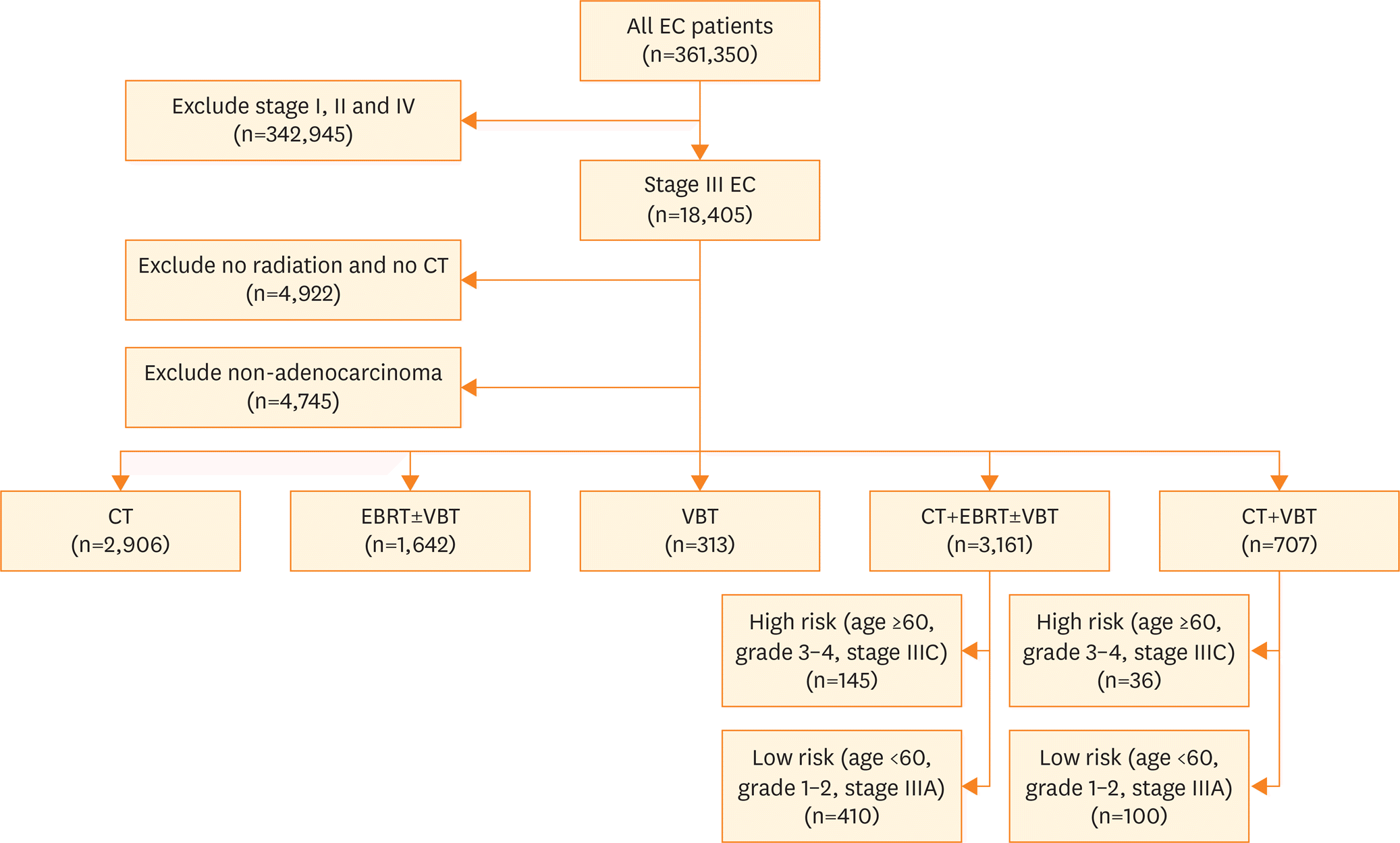 | Fig. 1.CONSORT diagram of patients with stage III endometrioid adenocarcinoma from NCDB receiving adjuvant therapy. CT, chemotherapy; EBRT, external beam radiotherapy; EC, endometrial cancer; NCDB, National Cancer Data Base; RT, radiation therapy; VBT, vaginal brachytherapy. |
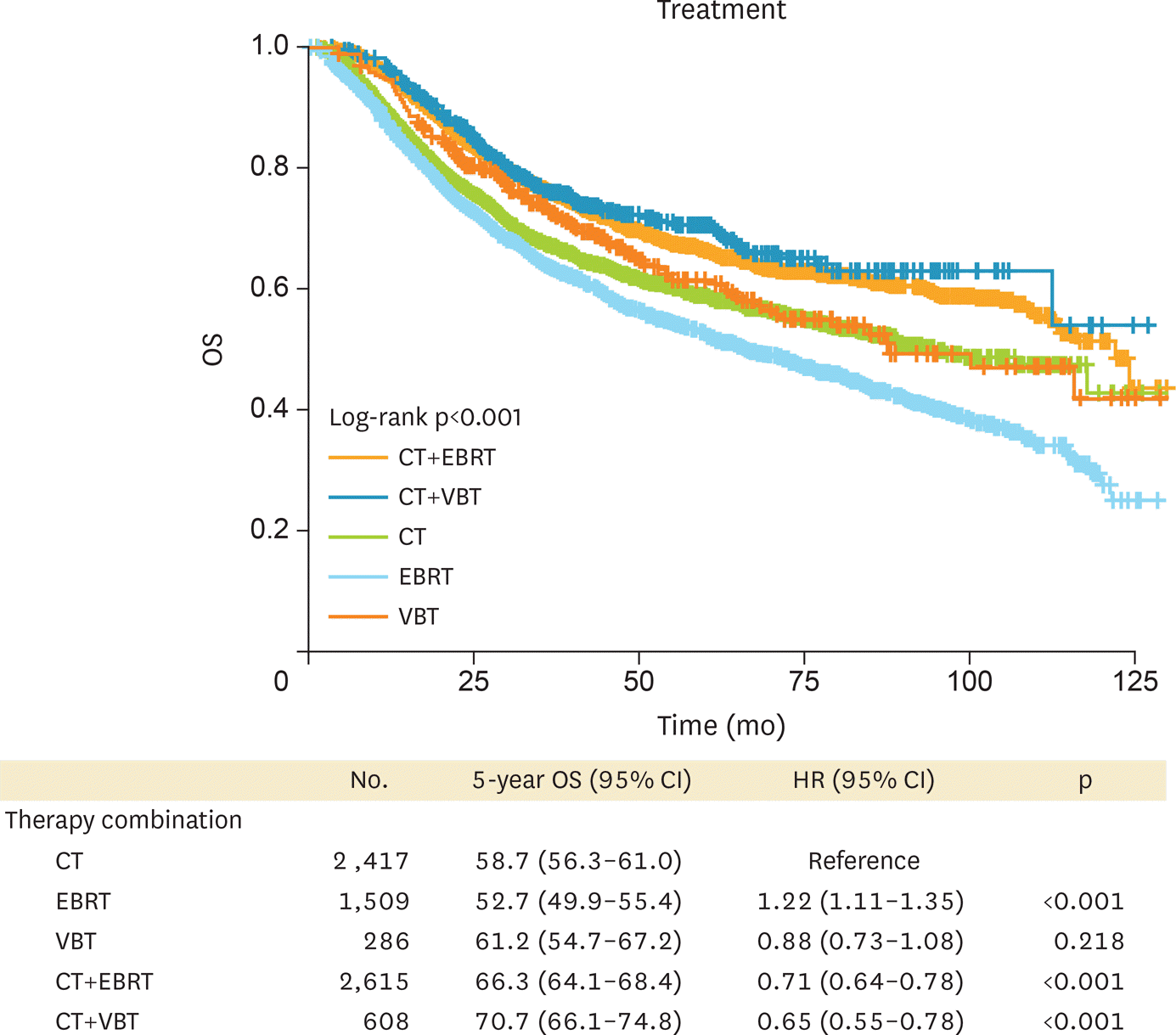 | Fig. 2.Survival of patients with LA-EAC according to adjuvant treatment modality. CMT with either EBRT or VBT had better survival compared to monotherapy. CI, confidence interval; CMT, combined modality treatment; CT, chemotherapy; EBRT, external beam radiotherapy; HR, hazard ratio; LA-EAC, locally advanced endometrioid adenocarcinoma; OS, overall survival; VBT, vaginal brachytherapy. |
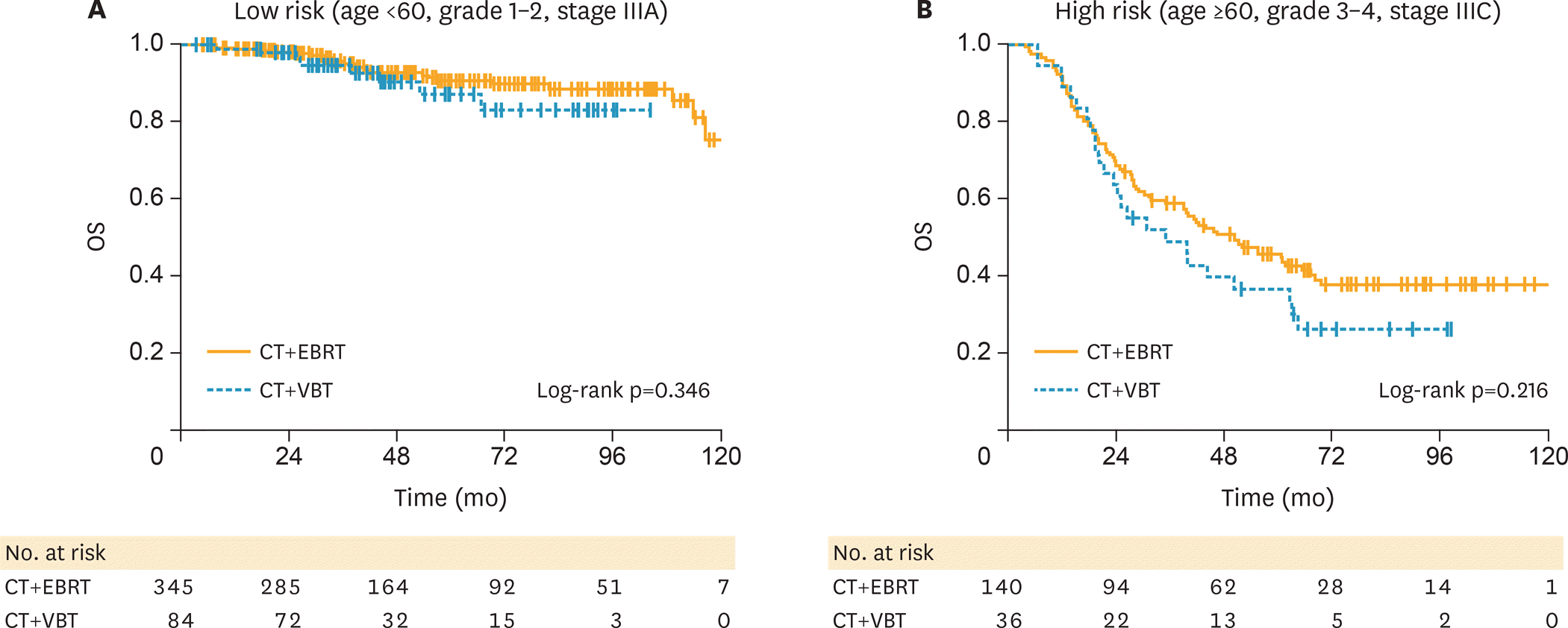 | Fig. 3.Survival of patients receiving adjuvant hemoradiation. (A) Low-risk (age <60, grade 1–2, stage IIIA) patients according to type of adjuvant radiation modality. (B) High-risk (age >60, grade 3–4, stage IIIC) patients according to type of adjuvant radiation modality. CT, chemotherapy; EBRT, external beam radiotherapy; OS, overall survival; VBT, vaginal brachytherapy. |
Table 1.
Demographics and clinical characteristics according to adjuvant CMT
Table 2.
Multivariate analysis of survival by CMT




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download