Abstract
The 2016 WHO diagnostic criteria for chronic myelomonocytic leukemia (CMML) require both absolute and relative monocytosis (≥1×109/L and ≥10% of white blood cell counts) in peripheral blood. Moreover, myeloproliferative neoplasm (MPN) features in bone marrow and/or MPN-associated mutations tend to support MPN with monocytosis rather than CMML. We assessed the impact of the 2016 WHO criteria on CMML diagnosis, compared with the 2008 WHO criteria, through a retrospective review of the medical records of 38 CMML patients diagnosed according to the 2008 WHO classification. Application of the 2016 WHO criteria resulted in the exclusion of three (8%) patients who did not fulfill the relative monocytosis criterion and eight (21%) patients with an MPN-associated mutation. These 11 patients formed the 2016 WHO others group; the remaining 27 formed the 2016 WHO CMML group. The significant difference in the platelet count and monocyte percentage between the two groups indicated that the 2016 WHO criteria lead to a more homogenous and improved definition of CMML compared with the 2008 WHO criteria, which may have led to over-diagnosis of CMML. More widespread use of molecular tests and more sophisticated clinical and morphological evaluations are necessary to diagnose CMML accurately.
Chronic myelomonocytic leukemia (CMML) is a clinically heterogeneous disorder with poor prognosis [1]. CMML, once classified as a MDS according to the French-American-British classification, is now recognized by the WHO classification as an overlap MDS/myeloproliferative neoplasm (MPN) [1]. The diagnosis of CMML can be difficult because it requires a combination of morphological, histopathological, and cytogenetic approaches [2]. MPN with monocytosis can simulate CMML in the absence of a previous history of MPN [3].
The 2016 WHO classification has resulted in several changes in the diagnosis and classification of CMML [4]. First, in contrast with the 2008 WHO classification [5], the diagnosis of CMML requires both the presence of absolute monocytosis (≥1×109/L) and relative monocytosis (≥10%) in peripheral blood (PB). Second, the presence of MPN features in the bone marrow (BM) and/or of MPN-associated mutations such as JAK2, CALR, and MPL, tend to support a diagnosis of MPN with monocytosis rather than CMML. Third, a further subdivision has split CMML-1 into CMML-0 and CMML-1. Fourth, CMML is divided into two subtypes—the proliferative type and the dysplastic type—based on white blood cell (WBC) counts.
The present study aimed to clarify the clinical and diagnostic significance of the 2016 WHO classification for CMML and to elucidate the current utilization status of molecular genetic profiles including MPN-associated mutations and BCR-ABL1 rearrangements.
We retrospectively reviewed the medical records of 38 CMML patients diagnosed according to the 2008 WHO criteria at seven university hospitals in Korea from January 2012 to July 2016. The laboratory data included a complete blood cell count with WBC differentials, BM morphology, a cytogenetic study, and a molecular genetic study including BCR-ABL1 and the mutational status of JAK2 V617F, JAK2 exon12, CALR, and MPL. In addition, an analysis for MPN-associated mutations was performed for 11 patients with available archival BM specimens. Allele-specific real-time PCR was used to detect JAK2 V617F. Sequencing was performed for both JAK2 exon12 and MPL W515. PCR/fragment analysis and sequencing was conducted for CALR exon 9. This study was approved by the Institutional Review Board of Ajou University Hospital.
Of the 38 CMML patients, three had <10% PB monocytes and eight had MPN-associated mutations according to the 2016 WHO criteria, which precluded their classification as CMML. These 11 patients were designated as the 2016 WHO others group and the remaining 27 as the 2016 WHO CMML group. The Mann-Whitney U test was used to compare the groups. Data were analyzed using SPSS 12.0.1 for Windows (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Table 1 shows the patients' demographic and laboratory characteristics. The significant difference between the two groups in platelet count and monocyte (%) level indicates that the 2016 WHO criteria offer a more homogenous and defined classification of CMML than the 2008 WHO criteria.
The 2016 WHO CMML group was subdivided according to the 2016 WHO classification as follows: 17 (63%) as CMML-0 (<2% blasts in PB and <5% blasts in BM), five (18.5%) as CMML-1 (2–4% blasts in PB and/or 5–9% blasts in BM), and five (18.5%) as CMML-2 (5–19% blasts in PB, 10–19% blasts in BM, and/or presence of any Auer rods). According to the 2008 WHO classification, the total samples were subdivided into 32 CMML-1 and six CMML-2 patients. Sixteen patients (59%) in the 2016 WHO CMML group had proliferative type CMML (WBC counts ≥13×109/L), and 11 (41%) had dysplastic type CMML (WBC counts <13×109/L). Most dysplastic type patients (9/11) had CMML-0. The dysplastic type within CMML-0 is known to have a better prognosis than other CMML subgroups [6]. Mild to moderate reticulin fibrosis in BM was present in 11 (69%) of the 16. None of the patients had a previous history of MPN.
Of the 38 CMML patients, MPN-associated mutations were found in eight (31%) of the 26 tested for the presence of ≥1 mutation (Table 2). All 27 patients in the 2016 WHO CMML group tested negative for BCR-ABL1. Utilization of MPN-associated mutations at diagnosis was very low, except for JAK2 V617F (Table 2). Clonal cytogenetic abnormalities, including trisomy 8, -Y, -7/del(7q), and trisomy 21, were present in eight (30%) of the 2016 WHO CMML group and three (27%) of the 2016 WHO others group. No patient had a chromosomal rearrangement involving 8p11, corresponding to the FGFR1 rearrangement, or t(8;9)(p22;p24.1), corresponding to PCM-JAK2. Clonal cytogenetic changes have been demonstrated in 20–40% of patients with CMML, but none are specific [37].
Recent studies have shown that at least one pathogenic mutation can be identified in >90% of CMML patients [89]. Gene mutations frequently observed in CMML include TET2, SRSF2, ASXL1, RAS, CBL1, and RUNX1. Next-generation sequencing for pathogenic mutations may be helpful for establishing a correct diagnosis in diagnostically difficult cases of CMML. In addition, a robust multiparameter flow cytometry assay could distinguish CMML from a diagnosis of reactive monocytosis and myeloid malignancies in patients with a borderline or increased monocyte count by detecting an increase in the fraction of classical CD14+/CD16− cells among the circulating monocytes [10].
Our data show that the rate of tests ordered for the detection of MPN-associated mutations at initial diagnosis is very low, except for the testing of JAK2 V617F. This was mainly because the national health insurance used to only cover JAK2 V617F testing. Currently, the national health insurance system in Korea covers all four MPN-associated mutations; thus, the usage rate is expected to increase. In addition, hematologists and hematopathologists need to make an effort to adhere to the 2016 WHO classification.
In conclusion, the 2008 WHO criteria may result in over-diagnosis of CMML compared with the 2016 WHO criteria. More aggressive use of molecular tests, including MPN-associated mutation analyses, and a more sophisticated clinical or morphological evaluation are necessary in order to achieve an accurate CMML diagnosis.
References
1. Itzykson R, Duchmann M, Lucas N, Solary E. CMML: clinical and molecular aspects. Int J Hematol. 2017; 105:711–719. PMID: 28455647.
2. Santini V, Allione B, Zini G, Gioia D, Lunghi M, Poloni A, et al. A phase II, multicenter trial of decitabine in higher-risk chronic myelomonocytic leukemia. Leukemia. 2018; 32:413–418. PMID: 28607470.
3. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127:2391–2405. PMID: 27069254.
4. Orazi A, Bennett JM, et al. Chronic myelomonocytic leukemia. In : Swerdlow SH, Campo E, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon: International Agency for Research on Cancer;2017. p. 82–86.
5. Orazi A, Bennett JM, et al. Chronic myelomonocytic leukemia. In : Swerdlow SH, Campo E, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer;2008. p. 76–79.
6. Schuler E, Schroeder M, Neukirchen J, Strupp C, Xicoy B, Kündgen A, et al. Refined medullary blast and white blood cell count based classification of chronic myelomonocytic leukemias. Leuk Res. 2014; 38:1413–1419. PMID: 25444076.
7. Patnaik MM, Tefferi A. Cytogenetic and molecular abnormalities in chronic myelomonocytic leukemia. Blood Cancer J. 2016; 6:e393. PMID: 26849014.
8. Elena C, Gallì A, Such E, Meggendorfer M, Germing U, Rizzo E, et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood. 2016; 128:1408–1417. PMID: 27385790.
9. Reinig E, Yang F, Traer E, Arora R, Brown S, Rattray R, et al. Targeted next-generation sequencing in myelodysplastic syndrome and chronic myelomonocytic leukemia aids diagnosis in challenging cases and identifies frequent spliceosome mutations in transformed acute myeloid leukemia. Am J Clin Pathol. 2016; 145:497–506. PMID: 27124934.
10. Selimoglu-Buet D, Wagner-Ballon O, Saada V, Bardet V, Itzykson R, Bencheikh L, et al. Characteristic repartition of monocyte subsets as a diagnostic signature of chronic myelomonocytic leukemia. Blood. 2015; 125:3618–3626. PMID: 25852055.
Table 1
Clinical and laboratory characteristics of patients diagnosed as having chronic myelomonocytic leukemia according to the WHO criteria
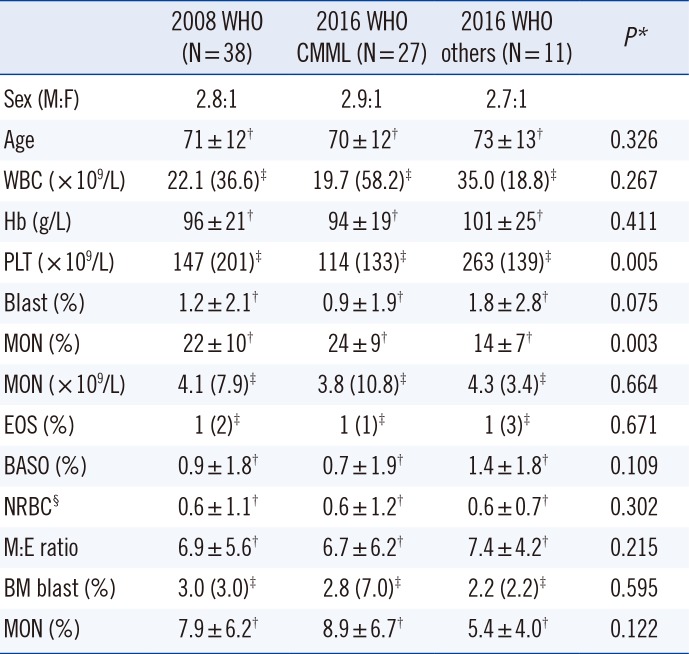
*The Mann-Whitney U test was used to compare the 2016 WHO CMML group and the 2016 WHO others group; †Values are presented as mean±SD; ‡Values are presented as median (interquartile range); §observed per 100 WBCs.
Abbreviations: CMML, chronic myelomonocytic leukemia; M:F, male to female ratio; WBC, white blood cells; PLT, platelets; MON, monocytes; EOS, eosinophils; BASO, basophils; NRBC, nucleated red blood cells; M:E, myeloid to erythroid; BM, bone marrow.
Table 2
Myeloproliferative neoplasm-associated mutations in 38 patients diagnosed as having chronic myelomonocytic leukemia according to the 2008 WHO classification
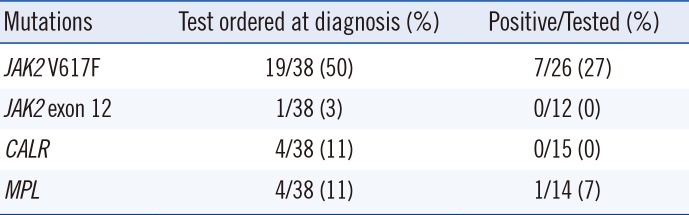
| Mutations | Test ordered at diagnosis (%) | Positive/Tested (%) |
|---|---|---|
| JAK2 V617F | 19/38 (50) | 7/26 (27) |
| JAK2 exon 12 | 1/38 (3) | 0/12 (0) |
| CALR | 4/38 (11) | 0/15 (0) |
| MPL | 4/38 (11) | 1/14 (7) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download