INTRODUCTION
The Epstein-Barr virus (EBV) is one of the most prevalent causes of viral infection in humans. EBV has been linked to infectious mononucleosis [
1]. Humans are the only natural hosts of EBV. Oral transmission is the primary route of infection in adolescents and young adults [
2]; however, EBV can also be acquired via blood transfusions, hematopoietic stem cell transplantation, or solid organ transplants, and these infections can be life-threatening [
3456].
In immunocompromised patients (particularly in solid organ transplant recipients), EBV DNA detection tests are performed within three months of transplantation and at least once per year in stable transplanted patients [
47]. However, these tests do not reflect EBV infection status in all patients, as the virus can exist in B lymphocytes and possess Herpesviridae latency [
8]. Antibody tests for EBV, such as those involving IgM and IgG antibodies against the EBV viral capsid antigen (VCA) and an IgG antibody against the EBV nuclear antigen (EBNA), are also frequently used to determine EBV infection status. EBV infection stage is typically assessed using a combination of antibody assays. An ideal EBV testing panel, including a combination of antibody assays for detecting EBV-specific antigens, should be able to detect all serologic infection statuses (acute, past, and absent infections).
Although the immunofluorescence assay (IFA) is considered the gold standard for the detection of VCA and EBNA IgG [
9], chemiluminescence immunoassays (CLIA) using automated analyzers are also widely used because of their objective interpretation of results, high throughput, and low labor intensiveness [
9101112131415]. Different commercial EBV antibody assays often show varying test results, thus hindering the interpretation of patient EBV infection status [
91016]. Reliable test results are needed for an exact diagnosis of EBV infection; therefore, EBV assays used in diagnostic laboratories must be evaluated for their accuracy, performance, and reliability.
Although many studies have compared commercial EBV assays [
91011121314], the diagnostic performance of the assays has not been evaluated using a standard EBV performance panel (SeraCare Life Science, Milford, MA, USA) covering various EBV infection status. To fill this gap, we evaluated the diagnostic performances of three commercial EBV antibody assays, Architect (Abbott Diagnostics, Wiesbaden, Germany), Liaison (DiaSorin, Saluggia, Italy), and Platelia (Bio-Rad, Marnes-la-Coquette, France) assays, using an EBV performance panel and compared the results of these three commercial assays in clinical samples of patients with suspected EBV infection.
Go to :

METHODS
1. Study samples
A total of 279 samples (from 123 males and 156 females with a median age of 25 years, range 11 months-96 years) for which an EBV PCR test and antibody assays (VCA IgM, VCA IgG, and EBNA IgG) were concurrently ordered, were collected from March 2015 to March 2016. All samples were derived from immunocompetent patients for the diagnosis of EBV infection. We excluded samples from immunocompromised patients (e.g., those with cancer, organ transplant recipients, or those with other infectious diseases). The Liaison assay was performed on the requested date for EBV antibody tests, and the residual samples were stored at –20℃, following separation for the Architect assay and Platelia assay and between assay testing. This study was approved by the Institutional Review Board (IRB) of Severance Hospital, Seoul, Korea. The IRB approved exemption for written informed consent, because this study was performed using stored residual samples after testing and based on a retrospective chart review.
2. EBV antibody assays
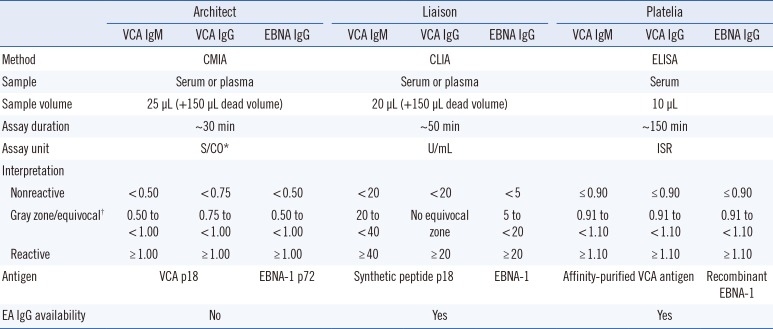
The Liaison assay was performed before the Architect and Platelia assays. The Liaison test is based on CLIA and samples were analyzed using the Liaison analyzer (DiaSorin); the Architect assay is a chemiluminescent microparticle immunoassay (CMIA) and was performed using the Architect i2000 analyzer (Abbott Diagnostics); and the Platelia test is an ELISA that was performed manually. Optical densities of the Platelia test were quantified using the BEP III System (Siemens, Marburg, Germany). For the Platelia assay, serum samples were diluted 1:21 (10 µL+ 200 µL) with the sample diluent provided with the assay kit. The instructions of the other tests did not recommend the use of serum dilution. All assay procedures followed the manufacturers' instructions (
Table 1).
Table 1
Characteristics of the three Epstein-Barr virus antibody assays
|
Architect |
Liaison |
Platelia |
|
VCA IgM |
VCA IgG |
EBNA IgG |
VCA IgM |
VCA IgG |
EBNA IgG |
VCA IgM |
VCA IgG |
EBNA IgG |
|
Method |
CMIA |
CLIA |
ELISA |
|
Sample |
Serum or plasma |
Serum or plasma |
Serum |
|
Sample volume |
25 µL (+150 µL dead volume) |
20 µL (+150 µL dead volume) |
10 µL |
|
Assay duration |
~30 min |
~50 min |
~150 min |
|
Assay unit |
S/CO*
|
U/mL |
ISR |
|
Interpretation |
|
|
|
|
|
|
|
|
|
|
Nonreactive |
<0.50 |
<0.75 |
<0.50 |
<20 |
<20 |
<5 |
≤0.90 |
≤0.90 |
≤0.90 |
|
Gray zone/equivocal†
|
0.50 to <1.00 |
0.75 to <1.00 |
0.50 to <1.00 |
20 to <40 |
No equivocal zone |
5 to <20 |
0.91 to <1.10 |
0.91 to <1.10 |
0.91 to <1.10 |
|
Reactive |
≥1.00 |
≥1.00 |
≥1.00 |
≥40 |
≥20 |
≥20 |
≥1.10 |
≥1.10 |
≥1.10 |
|
Antigen |
VCA p18 |
EBNA-1 p72 |
Synthetic peptide p18 |
EBNA-1 |
Affinity-purified VCA antigen |
Recombinant EBNA-1 |
|
EA IgG availability |
No |
Yes |
Yes |

3. EBV performance panel
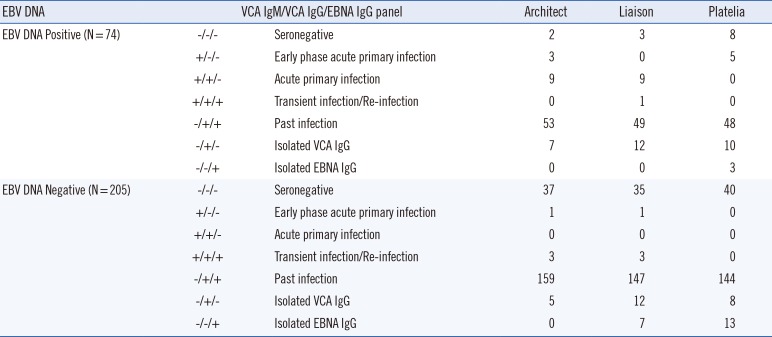
The Anti-Epstein-Barr Virus Mixed Titer Performance Panel (SeraCare Life Science) includes 21 samples covering different EBV infection status (one seronegative, 13 past infections [VCA IgM−, VCA IgG+, EBNA IgG+], three acute primary infections [VCA IgM+, VCA IgG+, EBNA IgG−], three early phase acute primary infections [VCA IgM+, VCA IgG−, EBNA IgG−], and one transient infection or re-infection [VCA IgM+, VCA IgG+, EBNA IgG+]) and a data sheet for comparing the panel results with the test results of the commercial EBV assays.
Lyophilized aliquots of donor plasma were dissolved in distilled water prior to analysis. Tests were performed in duplicate using the three commercial EBV assays to reduce the likelihood of experimental or reagent errors. VCA IgM values >43.9 and >1.0 were considered positive according to the performance panel data sheet of the Liaison and Platelia assays, respectively; VCA IgG values >1.0 were considered positive for the Platelia EBV assay; and EBNA IgG values >21.9 and >1.0 in the Liaison and Platelia EBV assays, respectively, were considered positive. The positive and negative results of the panel samples, obtained using the EBV antibody assays, were interpreted according to the performance panel instructions, and EBV antibody assay results were determined following the instructions of each assay.
4. EBV DNA test
PCR for EBV DNA was performed using the artus EBV RG PCR Kit (Qiagen, Hilden, Germany) on Rotor-Gene Q Instrument (Qiagen) after extracting DNA from plasma with the QIAamp DNA Mini Kit (Qiagen, Dusseldorf, Germany) according to the manufacturer's instruction.
5. Statistical analysis
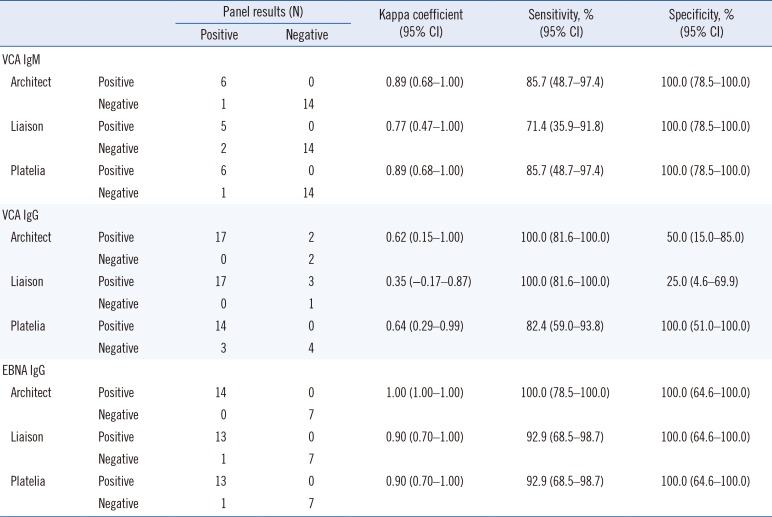
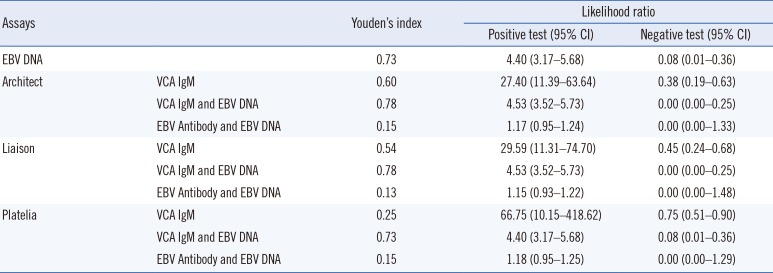
Kappa statistics, sensitivity, and specificity were calculated for VCA IgM, VCA IgG, and EBNA IgG based on the EBV performance panel results. We also calculated kappa statistics and the percentages of positive, negative, and total agreement between the Architect, Liaison, and Platelia EBV assays. Kappa values >0.75 indicated excellent agreement, values between 0.40 and 0.75 suggested fair to good agreement, and values <0.40 represented poor agreement. The percentage of total agreement between the assays represents the percentage of paired tests with identical results. Because “gray zone” or equivocal results cannot be interpreted as positive results and are mostly considered negative by clinicians, these results were grouped to evaluate kappa statistics and agreement percentages. The Student t-test was performed to determine the VCA IgM positive rate between the EBV DNA-positive and negative groups. P values<0.05 were considered statistically significant. To evaluate diagnostic performance based on clinical diagnosis of EBV infection of clinical samples, Youden's index and positive and negative Likelihood ratio were calculated. All statistical analyses were performed using Analyse-it, version 4.65 (Analyse-it Software, Ltd., Leeds, UK).
Go to :

DISCUSSION
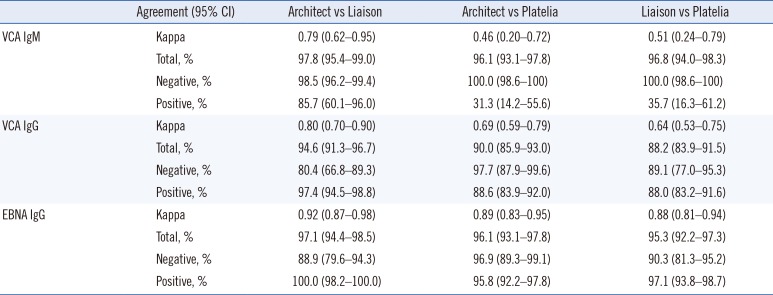
We evaluated the diagnostic performance of the Architect CMIA, Liaison CLIA, and Platelia ELISA EBV assays (VCA IgM, VCA IgG, and EBNA IgG antibodies) using an EBV performance panel as a reference. The EBV performance panel results indicated that the three EBV antibody assays showed excellent agreement for VCA IgM and EBNA IgG; however, the assays exhibited poorer VCA IgG detection than the performance panel. Although only a few VCA IgM-positive panel samples tested negative using the three antibody assays, false negative results could affect correct EBV infection diagnosis in patients with suspected EBV infection. Kappa agreement and sensitivity were significantly affected because of the limited number of VCA IgM-positive samples in the EBV performance panel. The bias of the performance panel towards more VCA IgM-negative and VCA IgG-positive and EBNA IgG-positive panel members could have caused different agreement results. One EBNA IgG-positive panel sample tested negative using the Liaison and Platelia assays.
This panel sample had a past infection status, and the test result was close to the cut-off value based on the EBV performance panel data sheet (Liaison, 33.15 U/mL; Platelia, 1.4 ISR). For VCA IgG, the Architect and Liaison assays showed positive results for two and three VCA IgG-negative panel members, respectively. In contrast, the Platelia assay demonstrated negative results for three VCA IgG-positive panel samples. This was likely due to differences in detection sensitivity between the assay methods used in the Architect and Liaison assays (CLIA vs ELISA).
As the Architect and Liaison assays showed more differences in their test results than the Platelia assay compared with the performance panel, the kappa agreement and specificity of the Architect and Liaison assays were affected more than those of the Platelia assay. The percentages of total agreement were 90.5%, 85.7%, and 85.7% between the EBV performance panel and the Architect, Liaison, and Platelia assays, respectively. However, false-positive and false-negative results have different importance in the field of infection diagnosis; Platelia assay false-negative cases represented the possibility of missed EBV infection diagnosis. We hypothesize that the performance panel had included more VCA IgM-positive and VCA IgG-negative samples, we would have seen excellent agreement and comparable sensitivity and specificity for the three EBV antibody assays.
When we compared the EBV DNA and VCA IgM antibody results, we detected significantly higher positivity rates for the EBV DNA test. The EBV DNA detection method has a higher sensitivity than serological antibody detection methods and EBV DNA can be detected even without EBV infection, as once infected with EBV, the virus might be latently present in B lymphocytes [
8]. Over 80% of clinical samples that tested negative for EBV infection using the antibody assays exhibited EBV DNA positivity. These samples were possibly collected during early infection stages (prior to VCA IgM formation); DNA positivity could also be due to EBV latency in B lymphocytes. In addition, EBV DNA detection can fail at times, despite current EBV infection, because of Herpesviridae latency in molecular testing [
8]. This could explain the VCA IgM positivity and EBV DNA negativity in a number of samples in our study. An evaluation of the diagnostic performance of the EBV DNA test and EBV antibody assays alone or in combination based on the clinical diagnosis of 279 patients indicated that the performance of the VCA IgM and EBV DNA combination was superior to that of the Architect or Liaison assay alone. Although most EBV DNA-negative samples were also VCA IgM negative and EBV DNA was detected in VCA IgM positive samples, the EBV DNA and antibody tests did not always show the same results. Therefore, EBV DNA and antibody assays should be used complementarily to diagnose the EBV infection status of patients.
The Architect and Liaison assays showed excellent agreement for VCA IgM, VCA IgG, and EBNA IgG. The Platelia assay showed more negative test results for VCA IgM- and VCA IgG-positive panel samples than the Architect and Liaison assays. This difference in sensitivity between the Platelia and the Architect and Liaison assays is most likely due to the fact that the Platelia assay uses ELISA, while the Architect and Liaison assays use CMIA and CLIA (with fully-automated analyzers), respectively. However, all kappa coefficients were >0.40; thus, the three EBV assays were considered to have fair and excellent agreement. The agreements between the different EBV antibody assays were comparable with the results of other studies [
910111213141517].
Our study has several limitations. First, the EBV performance panel and clinical samples were not tested with the IFA method, which is considered the gold standard for VCA IgG and EBNA IgG detection. However, excellent sensitivity and specificity have been reported for chemiluminescent EBV antibody detection assays [
12]. In addition, we used a commercial mixed performance panel consisting of various samples of EBV infection status to evaluate the diagnostic performance of the EBV antibody assays. The standard performance panel, which has been validated with several widely used EBV antibody assays and known EBV infection status, is a valuable evaluation tool for EBV antibody detection assays. Second, the study included only a few VCA IgM positive clinical and performance panel samples. Therefore, evaluating the accuracy of positive results for the VCA IgM assays was difficult and the agreement values between the different assays were likely affected. As EBV infection mostly occurs during childhood and adolescence and young patients typically do not show severe symptoms, the number of samples with new infection status was limited. Finally, we did not compare the EBV DNA test with the performance panel because of insufficient material. This may help explain the discrepancies in test results between the EBV DNA and antibody tests. Future studies including a sufficient number of samples with validated EBV infection status should be conducted to address this discrepancy and to accurately evaluate the diagnostic performance of the EBV assays.
Despite these limitations, to the best of our knowledge, this is the first study to evaluate the diagnostic performance of the Architect, Liaison, and Platelia EBV antibody assays using a standard performance panel. The diagnostic performances of the three EBV antibody assays using a combination of VCA IgM, VCA IgG, and EBNA IgG antibodies were acceptable. In addition, the three EBV antibody assays showed good agreement for the clinical samples. A combination of the EBV DNA and VCA IgM antibody tests would provide better diagnostic performance for patients with suspected EBV infection.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download