This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The urinary albumin/creatinine ratio (ACR) is an important indicator of albuminuria. We aimed to estimate ACR uncertainty and its impact on test results and proposed imprecision quality goals based on the estimated uncertainty.
Methods
The combined ACR uncertainty was calculated using the individual uncertainties of urinary albumin and creatinine. ACR confidence intervals (CIs) were estimated based on the expanded uncertainty. When the CI contained the ACR category boundary (30 or 300 mg/g), the cases were considered ambiguous. Quality goals for ACR were suggested using the number of ambiguous cases among actual patient results.
Results
The number of ambiguous cases resulting from the combined ACR uncertainty was higher than expected based on biological variation (BV) quality goals. When the ACR met BV quality specifications, we estimated that 4.8–15.5% of the results may have been misclassified. To minimize the number of ambiguous results, the minimum, desirable, and optimum quality goals were set at 34.0%, 18.0%, and 4.5%, respectively.
Conclusions
We expressed ACR uncertainty using the uncertainties of urinary albumin and creatinine and assessed the impact of this combined uncertainty on the test results. Subsequently, we proposed imprecision quality goals for ACR based on ambiguous results.
Keywords: Albumin/creatinine ratio, Uncertainty, Reclassification, Quality goal
INTRODUCTION
Monitoring the presence and/or progression of albuminuria is one of the most important clinical practices in various conditions [
1], including cardiovascular disease, for which it is a well-established risk factor [
2]. Although 24-hour urine collection is considered the gold standard method for detecting albuminuria, it requires considerable time and effort and its clinical utility is no better than calculating the urinary albumin/creatinine ratio (ACR) [
34]. Hence, the American Diabetes Association recommends that all patients with diabetes undergo screening for ACR, serum creatinine, and potassium [
5].
Accurate and precise measurements of both urinary albumin and creatinine are crucial for calculating ACR. However, combining the two measurements is likely to increase ACR calculation uncertainty [
6]. The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) has suggested a value based on biological variation (BV) as an attractive approach for quality requirements [
7]; however, this approach has some limitations for urine sample measurements [
8]. In addition, previously proposed urinary albumin and/or creatinine measurement quality goal settings were based on cutting-edge techniques, which are less suitable [
910].
We therefore calculated ACR uncertainty using the uncertainties of the albumin and creatinine results. We also attempted to estimate the impact of ACR uncertainty on actual test results and proposed quality standards for urinary ACR, urinary albumin, and creatinine test imprecisions.
METHODS
1. Data collection
All data were collected retrospectively. To calculate ACR, we consecutively collected and analyzed urine samples from patients at Dongtan Sacred Heart Hospital, Korea, from January to December 2016. Albumin and creatinine levels were analyzed using a Roche Cobas c702 analyzer with CREJ2 and ALBT2 reagents (Roche Diagnostics, Basel, Switzerland). The results were classified (according to ACR) into categories A1, A2, and A3, representing ACR<30 mg/g, 30–300 mg/g, and >300 mg/g, respectively [
11].
A total of 9,018 urine samples (male: 5,164, female: 3,854) were collected in order to calculate ACR. Of these, 150 samples (1.7%) were from children (<18 years). Based on albuminuria categories, 5,752 (63.8%) results were classified as A1 (<30 mg/g), 2,842 (31.5%) as A2 (30–300 mg/g), and 424 (4.7%) as A3 (>300 mg/g).
This study was approved by the Institutional Review Board of Dongtan Sacred Heart Hospital (2017-06-123). As this study is a retrospective analysis, no informed consent was obtained.
2. Calculation of ACR uncertainty
We used established formulae for calculating fractional uncertainty in variables [
6] to express the combined ACR uncertainty using the uncertainties of individual variables, as follows:
where
u is the standard uncertainty,
u(
ACR) is the uncertainty of ACR,
u(
ACR)/
ACR is the fractional uncertainty of ACR,
u(
Alb) is the uncertainty of urinary albumin,
u(
Alb)/
Alb is the fractional uncertainty of urinary albumin,
u(
Cr) is the uncertainty of urinary creatinine, and
u(
Cr)/
Cr is the fractional uncertainty of urinary creatinine.
3. Assessment of impact on patient results
To calculate the expanded ACR uncertainty and the corresponding 95% confidence interval (CI), the combined ACR uncertainty was multiplied by a coverage factor of two [
12]. If the CI of an individual patient result contained a classification boundary (30 mg/g or 300 mg/g), it was deemed to have potential for reclassification because of uncertainty. For example, a patient ACR result of 35 mg/g and a combined uncertainty of 12.8% would have an expanded uncertainty of 25.6% (12.8%×2). The 95% CI of the ACR result was calculated as [26.04, 43.96]. In this case, we considered the result as ambiguous, or “possible reclassification because of uncertainty.” A more detailed explanation of the computation can be found in
Supplemental Data S1.
The number of determined ambiguous cases was calculated based on ACR uncertainty; the impact of urinary albumin and creatinine measurement imprecision was assessed by counting the number of ambiguous cases when the two met the BV-derived quality requirements [
1314].
4. Proposed quality standards
ACR quality standards were proposed based on the number of ambiguous test results. There are no clear guidelines regarding the number of ambiguous cases acceptable for clinical purposes. Therefore, we referred to the number suggested by the original BV paper [
14]. Imprecision quality goals set by BV resulted in a 25% (minimum), 12% (desirable), and 3% (optimum) increase in result variability. Uncertainty levels generating the same number of ambiguous results as the BV-derived imprecision goals were proposed as the minimum, desirable, and optimum quality goals.
RESULTS
1. Assessment of ACR uncertainty and establishment of quality goals
Table 1 shows the combined fractional uncertainty of ACR when urinary albumin and creatinine uncertainties met the quality goals for variables subject to BV. As expected, the combined fractional uncertainty was notably higher than the two individual uncertainties.
2. Possible reclassification of test results
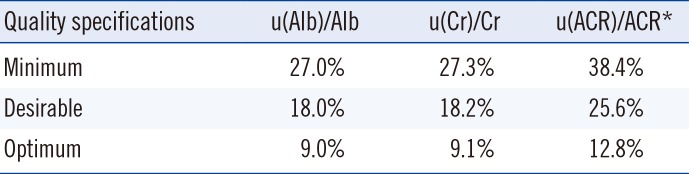
A possible reclassification of the albuminuria categories is shown in
Table 2. When ACR uncertainty met the optimum quality criteria in
Table 1, 4.8% (N=432) of the results were determined as possible ambiguous cases. However, this proportion increased to 15.5% (N=1,396) when the results were compared with the minimum quality requirements, as the fractional uncertainty of ACR increased.
Although category A3, known as macroalbuminuria, has been considered as a stage of irreversible kidney damage, several indications suggested that A2 category patients (microalbuminuria) could undergo regression [
151617]. Thus, we focused on the A2 category as the main target of ACR monitoring and calculated the ambiguous cases in this category.
The intermediate group (A2) is of great interest to help monitor disease progression in the clinical practice. The number of category A2 ambiguous cases was 808 (28.4%), 499 (17.6%), or 239 (8.4%), when ACR uncertainty met the minimum, desirable, or optimum quality criteria, respectively. Interestingly, the number of cases of possible reclassification to a lower category was higher than those of reclassification to a higher category. This might be due to the distribution of the original results (
Fig. 1), which exhibited a skewed distribution towards lower values in category A2.
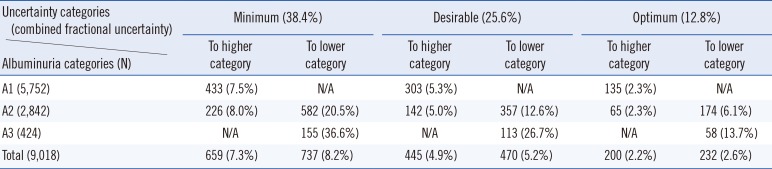
3. Proposed quality goals
The ACR quality goals that would limit the percentage of ambiguous cases to below 3%, 12%, and 25% are shown in
Table 3. For clinical reasons, it is especially important to monitor the progress of patients in category A2. Therefore, in order to minimize the number of ambiguous cases in this category, we proposed that the optimum quality goal for combined ACR uncertainty should be 4.5%, while the minimum should be up to 34%. All goals were lower than the calculated uncertainty shown in
Table 1.
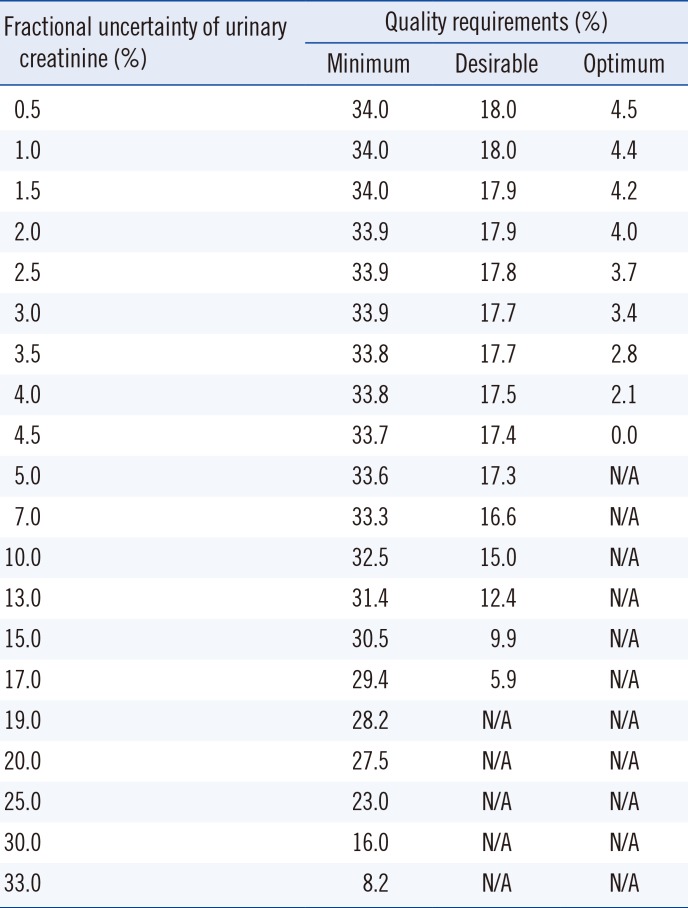
The relationships between urinary albumin and creatinine uncertainties are shown in
Table 4; an increase in the fractional uncertainty of urinary creatinine led to a decrease in the quality requirement for urinary albumin, and vice versa (
Table 4).
DISCUSSION
Our major findings are that 1) the number of ambiguous cases resulting from the combined ACR uncertainty was higher than expected based on the BV quality goals; and 2) it is feasible to set quality goals for combined/individual uncertainties based on the number of ambiguous cases.
Appropriate quality management is an essential requirement in clinical laboratories. Although many methods, such as expert consensus or clinicians' questionnaires, for setting quality goals have been suggested, according to the IFCC, the ideal goals should be based on clinical outcomes [
7]. However, because of a lack of tests with such goals, quality goals for variables subject to BV have been highlighted as appealing alternatives. Furthermore, the application of these specifications in urinary measurements is also limited [
8].
Imprecision goals for variables subject to BV and calculated based on uncertainty values have resulted in increased test variabilities: 25% (minimum), 12% (desirable), and 3% (optimum) [
14]. However, we revealed that the traditional quality goals for variables subject to BV had limited utility when applied to calculated parameters such as ACR. Various parameters are typically calculated from clinical laboratory measurementsIt is important to consider the effects of combined uncertainty on other results calculated from combined values, particularly when a clinical decision is based on the combined result.
There have been several attempts to express the uncertainties of calculated values using those of the measured values [
1218]. The most actively studied parameter is estimated glomerular filtration rate (eGFR); studies have suggested that the uncertainty of low creatinine levels, in particular, may affect the reliability of the eGFR value [
1218]. To the best of our knowledge, however, no previous studies have calculated ACR uncertainty. Our results show that it is feasible to estimate ACR uncertainty using the uncertainties of urinary albumin and creatinine levels.
Our study had two main limitations. First, we used test results from only one teaching hospital. Some slight variation in numerical values may be possible with a larger cohort of patients. However, as ACR estimates are used in clinical practice worldwide, it is unlikely that there would be substantial variation in the test result distribution in different clinical settings. Second, the covariance between urinary albumin and creatinine levels was not taken into account in our analysis. Any covariance between these two parameters would likely increase the combined uncertainty. Further investigation of this issue is recommended, as it was beyond the scope of our current study.
We attempted to propose a method for setting quality goals based on patient results. However, not all tests performed in clinical laboratories can be subject to such an approach. To apply this method, a clinical decision and/or clinical classification should be performed based solely on the test results. In other cases where clinical information is crucial for clinical management, laboratory results may play a supportive role.
In conclusion, we expressed ACR uncertainty using the uncertainties of urinary albumin and creatinine levels. We also assessed the impact of the combined uncertainty on test results using their variability. Quality goals for ACR calculation could be set based on test result ambiguities; using our results, we suggest an approach for setting quality goals based on clinical significance.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
1. Lambers Heerspink HJ, Gansevoort RT. Albuminuria is an appropriate therapeutic target in patients with CKD: the Pro View. Clin J Am Soc Nephrol. 2015; 10:1079–1088. PMID:
25887073.
2. Currie G, Delles C. Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis. 2013; 7:13–24. PMID:
24379690.
3. Rodby RA. Timed urine collections for albumin and protein: “The King Is Dead, Long Live the King!”. Am J Kidney Dis. 2016; 68:836–838. PMID:
27646424.
4. Talreja H, Akbari A, White CA, Ramsay TO, Hiremath S, Knoll G. Predicting kidney transplantation outcomes using proteinuria ascertained from spot urine samples versus timed urine collections. Am J Kidney Dis. 2014; 64:962–968. PMID:
25304983.
5. American Diabetes Association. Standards of medical care in diabetes-2017: summary of revisions. Diabetes Care. 2017; 40(S1):S4–S5. PMID:
27979887.
6. Farrance I, Frenkel R. Uncertainty of measurement: a review of the rules for calculating uncertainty components through functional relationships. Clin Biochem Rev. 2012; 33:49–75. PMID:
22896744.
7. Sandberg S, Fraser CG, Horvath AR, Jansen R, Jones G, Oosterhuis W, et al. Defining analytical performance specifications: consensus Statement from the 1st Strategic Conference of the European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem Lab Med. 2015; 53:833–835. PMID:
25719329.
8. Ceriotti F, Fernandez-Calle P, Klee GG, Nordin G, Sandberg S, Streichert T, et al. Criteria for assigning laboratory measurands to models for analytical performance specifications defined in the 1st EFLM Strategic Conference. Clin Chem Lab Med. 2017; 55:189–194. PMID:
27506603.
9. Shephard MD, Gill JP. An innovative Australian point-of-care model for urine albumin: creatinine ratio testing that supports diabetes management in indigenous medical services and has international application. Ann Clin Biochem. 2005; 42:208–215. PMID:
15949156.
10. Miller WG, Seegmiller JC, Lieske JC, Narva AS, Bachmann LM. Standardization of urine albumin measurements: status and performance goals. J Appl Lab Med. 2017; 2:423–429.
11. Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014; 85:49–61. PMID:
24284513.
12. Cavalier E, Ferir AM, Delanaye P, Krzesinski JM, Chapelle JP. Measurement uncertainty of creatinine in low values: another good reason not to use the MDRD formula with low creatinine values. Clin Biochem. 2007; 40:285–286. PMID:
17208211.
13. Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999; 59:491–500. PMID:
10667686.
14. Fraser CG, Hyltoft Petersen P, Libeer JC, Ricos C. Proposals for setting generally applicable quality goals solely based on biology. Ann Clin Biochem. 1997; 34:8–12. PMID:
9022883.
15. de Boer IH, Afkarian M, Rue TC, Cleary PA, Lachin JM, Molitch ME, et al. Renal outcomes in patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol. 2014; 25:2342–2350. PMID:
24925722.
16. de Boer IH, Rue TC, Cleary PA, Lachin JM, Molitch ME, Steffes MW, et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med. 2011; 171:412–420. PMID:
21403038.
17. Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003; 348:2285–2293. PMID:
12788992.
18. Parry D. Measurement uncertainty of eGFR at low creatinine levels: be careful to not overestimate. Clin Biochem. 2010; 43:349–350. PMID:
19766615.
Fig. 1
Histogram of urinary albumin/creatinine ratio (ACR) results in category A2 (30–300 mg/g of ACR). It showed a skewed distribution towards lower values.
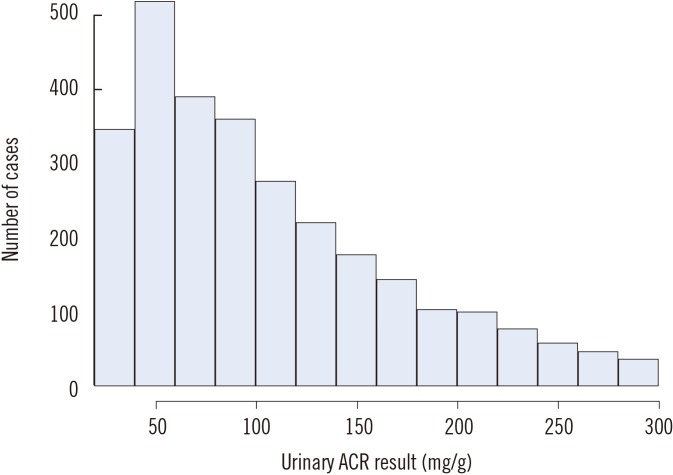
Table 1
Combined fractional uncertainty of the albumin/creatinine ratio when the urinary albumin and creatinine results meet the quality requirements for variables subject to biological variation
|
Quality specifications |
u(Alb)/Alb |
u(Cr)/Cr |
u(ACR)/ACR*
|
|
Minimum |
27.0% |
27.3% |
38.4% |
|
Desirable |
18.0% |
18.2% |
25.6% |
|
Optimum |
9.0% |
9.1% |
12.8% |
Table 2
Possible reclassification of albuminuria categories according to increased uncertainty of urinary ACR
|
Uncertainty categories (combined fractional uncertainty) |
Minimum (38.4%) |
Desirable (25.6%) |
Optimum (12.8%) |
|
Albuminuria categories (N) |
To higher category |
To lower category |
To higher category |
To lower category |
To higher category |
To lower category |
|
A1 (5,752) |
433 (7.5%) |
N/A |
303 (5.3%) |
N/A |
135 (2.3%) |
N/A |
|
A2 (2,842) |
226 (8.0%) |
582 (20.5%) |
142 (5.0%) |
357 (12.6%) |
65 (2.3%) |
174 (6.1%) |
|
A3 (424) |
N/A |
155 (36.6%) |
N/A |
113 (26.7%) |
N/A |
58 (13.7%) |
|
Total (9,018) |
659 (7.3%) |
737 (8.2%) |
445 (4.9%) |
470 (5.2%) |
200 (2.2%) |
232 (2.6%) |
Table 3
Proposed quality goals for the urinary albumin/creatinine ratio to limit ambiguous albuminuria category A2 cases to 25% (minimum), 12% (desirable), and 3% (optimum)
|
Uncertainty categories |
Minimum |
Desirable |
Optimum |
|
With proposed quality goals |
34.0% |
18.0% |
4.5% |
|
Expected ambiguous cases |
24.6% |
11.8% |
3.0% |
Table 4
Quality requirements for urinary albumin according to increasing urinary creatinine uncertainty
|
Fractional uncertainty of urinary creatinine (%) |
Quality requirements (%) |
|
Minimum |
Desirable |
Optimum |
|
0.5 |
34.0 |
18.0 |
4.5 |
|
1.0 |
34.0 |
18.0 |
4.4 |
|
1.5 |
34.0 |
17.9 |
4.2 |
|
2.0 |
33.9 |
17.9 |
4.0 |
|
2.5 |
33.9 |
17.8 |
3.7 |
|
3.0 |
33.9 |
17.7 |
3.4 |
|
3.5 |
33.8 |
17.7 |
2.8 |
|
4.0 |
33.8 |
17.5 |
2.1 |
|
4.5 |
33.7 |
17.4 |
0.0 |
|
5.0 |
33.6 |
17.3 |
N/A |
|
7.0 |
33.3 |
16.6 |
N/A |
|
10.0 |
32.5 |
15.0 |
N/A |
|
13.0 |
31.4 |
12.4 |
N/A |
|
15.0 |
30.5 |
9.9 |
N/A |
|
17.0 |
29.4 |
5.9 |
N/A |
|
19.0 |
28.2 |
N/A |
N/A |
|
20.0 |
27.5 |
N/A |
N/A |
|
25.0 |
23.0 |
N/A |
N/A |
|
30.0 |
16.0 |
N/A |
N/A |
|
33.0 |
8.2 |
N/A |
N/A |









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download