Abstract
Chronic migraine (CM) is a common and disabling neurologic disorder. CM is defined as more than 15 days a month over a 3-month period, including at least 8 days per month on which their headaches and associated symptoms meet diagnostic criteria for migraine. Quality of life is highly compromised in patients with this condition, and comorbidities are more frequent than with episodic migraine. The diagnosis requires a carefully-conducted patient interview and neurologic examination, sometimes combined with additional diagnostic tests, to differentiate CM from secondary headache disorders and other primary chronic headaches. CM typically develops from episodic migraine over months to years. Several factors are associated with an increased risk of episodic migraine developing into CM, including the frequent use of abortive migraine drugs. Through identification of risk factors for progression to CM, clinicians can educate patients about modifiable risk factors and can begin appropriate individualized preventive therapy. There is a high frequency of medication overuse in CM. The first step in the management of CM complicated by medication overuse is withdrawal of the overused drugs and detoxification treatment. This article provides an overview of CM, including its epidemiology, risk factors for its development, and information on its pathophysiology, diagnosis, and management.
Go to : 
REFERENCES
1. Natoli JL, Manack A, Dean B, Butler Q, Turkel CC, Stovner L, Lipton RB. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010; 30:599–609.

2. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; 38:1–211.
4. Schwedt TJ, Larson-Prior L, Coalson RS, Nolan T, Mar S, Ances BM, Benzinger T, Schlaggar BL. Allodynia and descending pain modulation in migraine: a resting state functional connectivity analysis. Pain Med. 2014; 15:154–165.

5. Cernuda-Morollon E, Larrosa D, Ramon C, Vega J, Martinez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. 2013; 81:1191–1196.

6. Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008; 48:1157–1168.

7. Scher AI, Midgette LA, Lipton RB. Risk factors for headache chronification. Headache. 2008; 48:16–25.

8. Bigal ME, Lipton RB. The differential diagnosis of chronic daily headaches: an algorithm-based approach. J Headache Pain. 2007; 8:263–272.

9. Tassorelli C, Jensen R, Allena M, De Icco R, Sances G, Katsarava Z, Lainez M, Leston J, Fadic R, Spadafora S, Pagani M, Nappi G. the COMOESTAS Consortium. A consensus protocol for the management of medication-overuse headache: evaluation in a multicentric, multinational study. Cephalalgia. 2014; 34:645–655.

10. Rossi P, Jensen R, Nappi G, Allena M. COMOESTAS Consortium. A narrative review on the management of medication overuse headache: the steep road from experience to evidence. J Headache Pain. 2009; 10:407–417.

11. Evers S, Jensen R. European Federation of Neurological Societies. Treatment of medication overuse headache–guideline of the EFNS headache panel. Eur J Neurol. 2011; 18:1115–1121.
12. Evers S, Marziniak M. Clinical features, pathophysiology, and treatment of medication-overuse headache. Lancet Neurol. 2010; 9:391–401.

13. Krymchantowski AV, Barbosa JS. Prednisone as initial treatment of analgesic-induced daily headache. Cephalalgia. 2000; 20:107–113.

14. Saper JR, Mathew NT, Loder EW, DeGryse R, VanDenburgh AM. BoNTA-009 Study Group. A double-blind, randomized, placebo-controlled comparison of botulinum toxin type a injection sites and doses in the prevention of episodic migraine. Pain Med. 2007; 8:478–485.

15. Aurora SK, Dodick DW, Turkel CC, DeGryse RE, Silberstein SD, Lipton RB, Diener HC, Brin MF. PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010; 30:793–803.

16. Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, Lipton RB, Diener HC, Brin MF. PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010; 50:921–936.

17. Mathew NT, Jaffri SF. A double-blind comparison of onabotulinumtoxina (BOTOX) and topiramate (TOPAMAX) for the prophylactic treatment of chronic migraine: a pilot study. Headache. 2009; 49:1466–1478.

18. Cady RK, Schreiber CP, Porter JA, Blumenfeld AM, Farmer KU. A multicenter double-blind pilot comparison of onabotulinumtoxinA and topiramate for the prophylactic treatment of chronic migraine. Headache. 2011; 51:21–32.

19. Magalhaes E, Menezes C, Cardeal M, Melo A. Botulinum toxin type A versus amitriptyline for the treatment of chronic daily migraine. Clin Neurol Neurosurg. 2010; 112:463–466.
20. Yurekli VA, Akhan G, Kutluhan S, Uzar E, Koyuncuoglu HR, Gultekin F. The effect of sodium valproate on chronic daily headache and its subgroups. J Headache Pain. 2008; 9:37–41.

21. Mathew NT, Rapoport A, Saper J, Magnus L, Klapper J, Ramadan N, Stacey B, Tepper S. Efficacy of gabapentin in migraine prophylaxis. Headache. 2001; 41:119–128.

22. Saper JR, Lake AE 3rd, Cantrell DT, Winner PK, White JR. Chronic daily headache prophylaxis with tizanidine: a double-blind, placebo-controlled, multicenter outcome study. Headache. 2002; 42:470–482.

23. Bigal M, Rapoport A, Sheftell F, Tepper D, Tepper S. Memantine in the preventive treatment of refractory migraine. Headache. 2008; 48:1337–1342.

24. Calandre EP, Garcia-Leiva JM, Rico-Villademoros F, Vilchez JS, Rodriguez-Lopez CM. Pregabalin in the treatment of chronic migraine: an open-label study. Clin Neuropharmacol. 2010; 33:35–39.
25. Ashkenazi A, Benlifer A, Korenblit J, Silberstein SD. Zonisamide for migraine prophylaxis in refractory patients. Cephalalgia. 2006; 26:1199–1202.

26. Aurora SK, Dodick DW, Diener HC, DeGryse RE, Turkel CC, Lipton RB, Silberstein SD. OnabotulinumtoxinA for chronic migraine: efficacy, safety, and tolerability in patients who received all five treatment cycles in the PREEMPT clinical program. Acta Neurol Scand. 2014; 129:61–70.
27. Silberstein SD, Lipton RB, Dodick DW, Freitag FG, Ramadan N, Mathew N, Brandes JL, Bigal M, Saper J, Ascher S, Jordan DM, Greenberg SJ, Hulihan J. Topiramate Chronic Migraine Study Group. Efficacy and safety of topiramate for the treatment of chronic migraine: a randomized, double-blind, placebo-controlled trial. Headache. 2007; 47:170–180.

28. Diener HC, Bussone G, Van Oene JC, Lahaye M, Schwalen S, Goadsby PJ. TOPMAT-MIG-201(TOP-CHROME) Study Group. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007; 27:814–823.

Go to : 
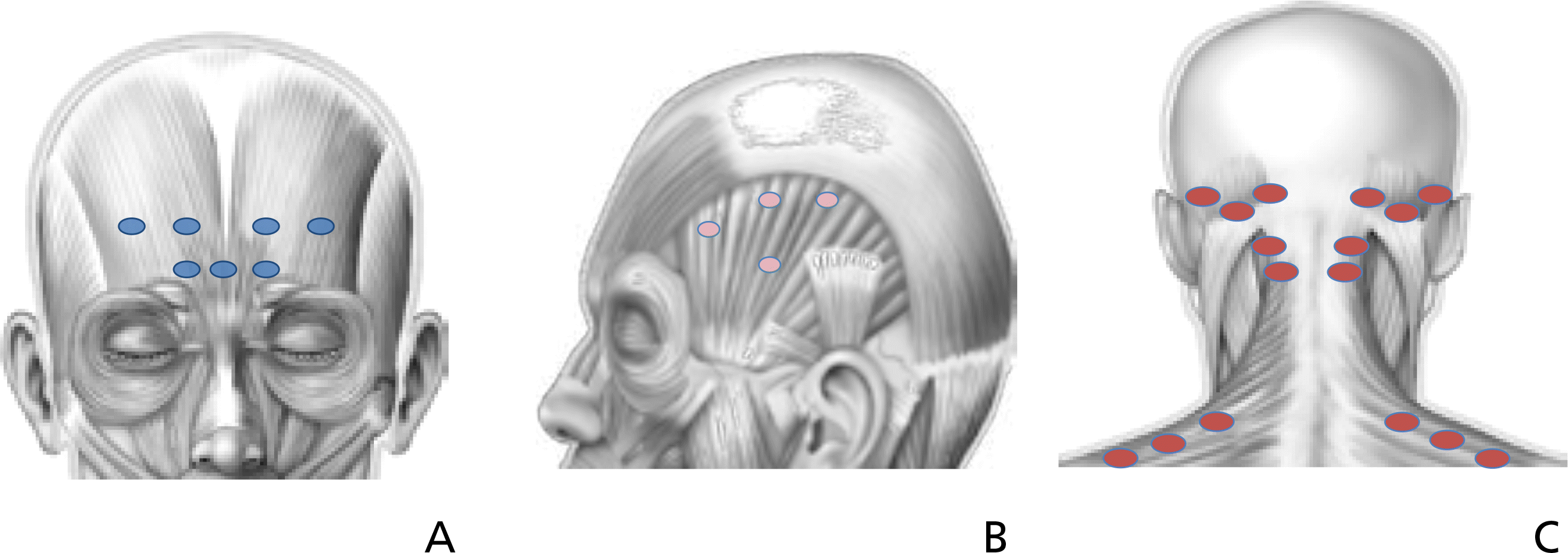 | Figure 2.Fixed sites in Phase 3 PREEMPT (Phase 3 Research Evaluating Migraine Prophylaxis Therapy) study. The anatomic injection sites follow distributions and areas innervated by the trigeminal nerve complex. (A) Anterior injection on corrugator, proceus, and frontalis. (B) Lateral injection on temporalis. (C) Posterior injection on occipitalis, cervical paraspinal, and trapezius. |
Table 1.
Chronic migraine diagnostic criteria [2]
Table 2.
Medication overuse headache diagnostic criteria [2]
Table 3.
Red flags suggestive of secondary headache disorders [8]
Table 4.
Drug prophylaxis of chronic migraine [3]
Table 5.
Most effective botulinum injection sites for chronic migraine (PREEMPT injection paradigm)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download