Abstract
Volumetric absorptive microsampling (VAMS) is a novel sampling technique that allows for the collection of an accurate volume of blood by dipping a microsampler tip. The purpose of this study is to compare the requirement of a stabilizing reagent for the conventional venous blood sampling method versus VAMS in the analytical measurement of the concentration of acetylsalicylic acid. A high-performance liquid chromatography with mass spectrometry (LC-MS/MS) method was developed and validated for the accurate determination of acetylsalicylic acid in human blood. The blood samples spiked with acetylsalicylic acid with and without stabilizing reagent were absorbed into VAMS tips. In the whole blood sample, the same concentration was shown regardless of the addition of the stabilizing reagent, but the concentration decreased when the stabilizing reagent was not added to the VAMS sample. To apply the VAMS technology as a new blood sampling method, stabilizing reagents should be added before the analysis of acetylsalicylic acid concentration.
Volumetric absorptive microsampling (VAMS) is a novel technique for the collection of an accurate blood volume to enable quantitative analysis in microsamples (typically within the range of 10–100 µL).[1] The Mitra® microsampler based on the VAMS is less invasive than the conventional venous whole blood sampling method because it can be dried, stored and transported in ambient conditions after blood sampling.[12] In the microsampling method, a fingertip is pierced with a lancet and the blood is absorbed by a Mitra microsampler. It has been reported that the collection of blood by absorbing a small volume of blood (10 µL or 20 µL) using VAMS allows for accurate quantification, and it is a device that allows for quantitative analysis independent of the hematocrit.[345]
Aspirin (acetylsalicylic acid) inhibits platelet aggregation through prevention of thromboxane A2. It is used for the prevention and treatment of various vascular diseases such as coronary artery disease, cerebrovascular disease, and peripheral vascular disease.[67] Acetylsalicylic acid also acts on the hypothalamus to produce anti-pyresis; heat dissipation is increased because of vasodilation and increased peripheral blood flow. The antipyretic activity of acetylsalicylic acid is also related to the inhibition of the synthesis and release of prostaglandins.[89] Acetylsalicylic acid is rapidly hydrolyzed to salicylic acid in the liver and small intestine and is then absorbed into the blood. [10] Salicylic acid is the compound that is primarily responsible for its pharmacological activity. Aspirin, a non-steroidal anti-inflammatory drug (NSAIDs), is also used for the prevention of myocardial infarction and stroke.[11] It is essential for the treatment of coronary artery disease such as acute coronary syndrome including stable angina pectoris or acute myocardial infarction and atherothrombotic diseases such as ischemic cerebral infarction, and it is effective for secondary prevention in a patient having a history of cardiovascular disease. Acetylsalicylic acid directly and irreversibly inhibits the activity of both types of cyclooxygenases (COX-1 and COX-2) to decrease the formation of precursors of prostaglandins and thromboxanes from arachidonic acid.[12] This makes acetylsalicylic acid different from other NSAIDS, which are reversible inhibitors. Salicylic acid may competitively inhibit prostaglandin formation.
Conventional methods for the analysis of aspirin levels in the blood includes detection of acetylsalicylic acid, which is the major component, and salicylic acid, which is its active form in the body. There are high-performance liquid chromatography (HPLC), capillary electrophoresis (CE) and liquid chromatography mass spectrometry (LC-MS/MS) methods for their detection in biological samples.[713141516] It is difficult to quantify the levels of acetylsalicylic acid in the blood when it is hydrolyzed to salicylic acid. However, the conversion to salicylic acid can be minimized by adding an enzyme inhibitor stabilizing reagent such as potassium fluoride or sodium fluoride.[1718]
If the VAMS method is introduced to patients or subjects in a clinical trial, the burden on the patient will be reduced by the minimal blood sampling. Therefore, we conducted a study on the accuracy of drug analysis using VAMS before applying it to a real clinical situation. We conducted a study on the assumption that acetylsalicylic acid, a drug that needs stabilization in blood analysis, can be quickly and easily collected and analyzed without the need for the addition of a stabilizing reagent by using a VAMS device. To investigate the effect of stabilizing reagent addition on the microsampler sample, we analyzed acetylsalicylic acid and salicylic acid, its hydrolyzed form.
Acetylsalicylic acid, hydrochlorothiazide, potassium fluoride and formic acid were all purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile and water were purchased at the highest grade available from Fisher Scientific (Seoul, Korea). All other reagents used were of analytical grade and were purchased from Sigma-Aldrich. Microsampler devices were purchased from Phenomenex (Neoteryx, Torrance, CA, USA, brand name Mitra®).
All samples were analyzed by LC-MS/MS using an HPLC 1100 series (Agilent, Applied Biosystems, USA) and an API 4000 QTRAP mass spectrometer (AB SCIEX, Applied Biosystems, Foster City, CA, USA) configuration. Chromatography and mass spectrometry parameters for microsampler samples and whole blood analysis are described elsewhere.[19] The instrument settings for the analysis of microsampler samples were identical to those for whole blood. The confirmation ion transitions for quantification were m/z 178.9→137.0 for acetylsalicylic acid and 295.9→78.1 for hydrochlorothiazide with an electrospray ionization interface used to generate negative ions [M − H]− in Figure 1.[20] Chromatographic separations were performed using a Luna, C18 column (2.0 × 50 mm, particle size 5 µm; Phenomenex, California, USA). The mobile phase consisted of 0.1% formic acid in 20% water and 0.1% formic acid in 80%acetonitrile. The flow rate was kept constant at 0.2 mL/min.
Volume tests were carried out to perform matrix absorption experiments by means of a balance with a resolution of 0.01 mg.[3] The control protocol for weight measurements was as follows: a 1 mL aliquot of the blood was placed in a vial, and to determine the weight (n = 6) of an accurately pipetted volume of each sample, this initial weight was recorded; then, 10 µL of sample was removed using a pipette, and the vial was finally reweighed. The average density of each matrix was calculated using the formula below:
The VAMS protocol for measurements of the volume absorbed by the VAMS device was as follows: to determine the weight (n = 6) of the blood absorbed by the VAMS tip, the initial weight of a vial containing 1 mL of the blood sample was recorded; a VAMS device was carefully dipped into the sample; and after filling the tip was removed, and the vial reweighed. The volume absorbed on the VAMS tip for blood was calculated using the formula below:
Acetylsalicylic acid concentrations were determined in whole blood samples and microsampler samples using blank whole blood originating from a heathy male subject. Acetylsalicylic acid free venous whole blood from an acetylsalicylic acid abstinent healthy volunteer was collected in ethylenediaminetetraacetic acid (EDTA) tubes and used to prepare calibrators and quality control (QC) samples. Whole blood samples were obtained by transferring 50 mL of blood to 2 mL tubes. Standard stock solutions of acetylsalicylic acid were prepared with 0.1% formic acid in 50% acetonitrile at 1 mg/mL. Standard solutions of acetylsalicylic acid in human plasma were prepared by spiking with an appropriate volume of the variously diluted stock solutions, giving final concentrations of 0.4, 0.8, 1, 4, 8 and 10 µg/mL. The calibration standards were prepared at concentrations of 40~1,000 ng/mL of acetylsalicylic acid by spiking 80 µL of human blood with 10 µL of each working standard and 10 µL of 50% potassium fluoride. The samples were mixed, and 10 µL of them was transferred to a new tube. Then, 10 µL of hydrochlorothiazide (10 µg/mL in 0.1% formic acid in 50% acetonitrile) and 250 µL acetonitrile were carefully added to each sample. For extraction, samples were sonicated for 15 minutes, vortexed for 30 s and centrifuged at 10,000 rpm for 10 minutes at 4℃. The supernatants were transferred to injection vials, and 5 µL was injected into an LC-MS/MS system for analysis.
Microsampler samples preparation was similar to the methods used for the whole blood samples. Microsampler samples were dipped into the samples that consisted of spiked 80 µL of human blood with 10 µL of each working standard and 10 µL of 50% potassium fluoride. Microsampler samples were generated by dipping the upper part of the tip into a volume of whole blood contained in 2 mL tubes, thereby ensuring that the tips were not completely immersed into the blood to prevent overfilling. Upon turning completely red, the tips were held in place for an additional 2 s. Subsequently, the devices were positioned in a dedicated rack to prevent samples from touching each other while being air-dried for 1 h at room temperature. Dried samples could be stored at room temperature in the presence of desiccant in a closed plastic box or a zip-closure plastic bag. Microsampler tips were separated from the handler and placed in 2 mL tubes before 250 µL of acetonitrile and 10 µL of internal standard was added. The tips were extracted for 15 minutes in a sonicator and centrifuged at 10,000 rpm for 10 minutes at 4℃. The supernatant was transferred to an LC vial, and 5 µL was injected into the LC-MS/MS column.
The validation of the LC-MS/MS method was performed for specificity, limit of detection (LOD), lower limit of quantification (LLOQ), linearity, precision, accuracy, and recovery parameters. The specificity of the method was investigated by comparing the chromatograms of extracted blank blood obtained from six different human blood samples spiked with acetylsalicylic acid to ensure that they were free of interference around the retention time of acetylsalicylic acid. The LOD was determined by diluting solutions of known concentration until the response was three times the noise (S/N ration: 3), and the LLOQ was defined as the lowest concentration that could be calculated based on a minimal accepted value of S/N ratio 10. The linearity of the method was shown with a calibration curve constructed using six concentration points. Calibration curves were constructed by plotting the response ratios versus concentration of each analyte using a linear least squares regression with weighting factors of 1/x. The precision study was carried out based on injection repeatability and analysis repeatability of the spiked blood samples. Injection repeatability was determined by repeated injections of blood samples spiked with standard mixtures equivalent to each analyte into the LC-MS/MS system. The accuracy of the method was defined by replicate analysis of samples containing known amounts of the analyte. The deviation of the mean from the true value served as the measure of accuracy. The recovery of the method was determined as the ratio of the peak area of extracted samples after a full assay to that of a direct injection of equivalent concentration.
To investigate the effect of stabilizing reagents, the differences between concentration of microsampler sample and concentration of whole blood sample were compared with and without 50% potassium fluoride. First, 10 µL of acetylsalicylic acid QC sample was added to 80 µL of blank blood sample and 10 µL of stabilizing reagent was added. The whole blood sample was pretreated as it was, and the microsampler sample was absorbed onto 10 µL microsampler tip, dried for 1 hour, and extracted with acetonitrile. For samples without stabilizing reagents, 10 µL of acetylsalicylic acid QC was added to 90 µL of blank blood sample. The whole blood sample was pretreated as it was, and the microsampler sample was absorbed onto the tip and pretreated.
The results for the sampled volume test of VAMS device are shown in Figure 2A, are the collected volumes are statistically indistinguishable from those pipetted (~10 µL), thus showing a good sampling accuracy by the VAMS devices. Blood were sampled until the VAMS tip was completely full (~2 s), and then the tip was kept in contact with each matrix for an additional 2, 4, 6, 8 and 10 s. As shown in Figure 2B, the microsampling was volumetrically accurate at ~10 µL and there was no additional effect due to longer exposure times with each matrix, thus avoiding the risk of over sampling. The operators were given a brief description of the device and its use and allowed up to two practice attempts prior to each taking eight replicate samples. The accuracy (bias) for individual operators ranged from 0.13% to 0.87%, with precision (% CV) in the range of 1.29–7.91% (Figure. 2C), with an average volume of 10.38 µL (CV 4.2%). Thus, in use, VAMS has similar volume errors to other already accepted and established techniques. It was noted that operator A had a cluster of VAMS samples where the volumes were considerably higher than the other operators. These outliers could have been caused by immersing the tip past the shoulder, with the mechanism by which the blood becomes trapped being discussed later. Deep immersion could have been possible since the blood pool (approximately 1 mL) was contained in a 1.5 mL Eppendorf tube. It should be noted that deep immersion of the tip is not possible with the shallower pools obtained when either a rodent tail or finger prick are sampled. The responses to questionnaires indicated that all of the operators found the device easy to use and that they knew intuitively when the tip was full. The intuitive nature of the VAMS technique, however, does not negate the need for suitable training, as indicated by the high spread of volumes obtained by operator A.
An LC-MS/MS method for the determination of acetylsalicylic acid in human blood was developed and validated. The representative chromatograms of acetylsalicylic acid (40 ng/mL) in blank blood, LLOQ, and IS are shown in Figure 3 for selectivity. The results of these analyses were used to evaluate the linearity range of quantification and LLOQ, set to the lowest point of the calibration curve. The whole blood was validated over a concentration range of 40~1,000 ng/mL. A good linearity was obtained with r always larger than 0.995. The precision of the determination was evaluated in both MS/MS acquisition methods according to the calculated percentage of the replicated extracts sample. The precision and accuracy results are reported in Table 1 and 2. Intra-day precision ranged from 0.9% to 5.4%, and intra-day accuracy (RE%) ranged from −8.3% to 9.6%. Interday precision ranged from 1.5% to 6.9%, and inter-day accuracy (RE%) ranged from −6.6% to 6.8%. The intra-day precision and accuracy for QC samples were 1.7~7.7% (CV) and 92.5~100.7%, and the inter day precision and accuracy were 3.8~7.0% (CV) and 92.9~99.8%, respectively. To evaluate these determinations, three points of the calibration curve (at low, medium, and high concentration) were processed by the previously mentioned protein precipitation extraction method and then analyzed in triplicate. No unacceptable interfering peaks were observed in microsampling samples prepared from blank blood from 6 individual sources and the IS did not contribute to the responses of acetylsalicylic acid. Furthermore, no carry-over was found in blank samples injected after the ULOQ (upper limit of quantification). The recovery was evaluated by processing samples with 40, 400 and 800 ng/mL acetylsalicylic acid concentration. The recovery is expressed by the percent recovery reported in Table 3. Recovery rates were >92.5%. The intra- and inter-day precisions and accuracies below 15% indicated that in stabilized human blood samples, acetylsalicylic acid concentrations can be determined with reasonable precision and accuracy and that these methods can be used for microsampler studies.
The stability data for LLOQ and high QCs, analyzed in triplicate, are presented in Table 4 and demonstrate that acetylsalicylic acid was stable in microsampler samples for at least 30 days when stored at −70℃ and for at least 30 minutes when stored at room temperature, the latter representing potential shipping conditions. Furthermore, processed samples were stable when stored for at least 24 hours in the autosampler set at 4℃ and for at least 5 hours in an ice bath. In the freeze-thaw stability test, samples were stable for at least 3 cycles, and the re-injected sample was stable for at least 24 hours.
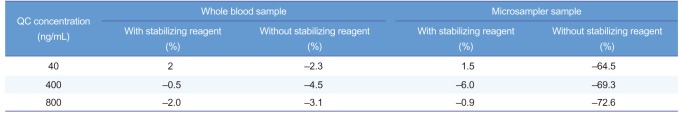
In the whole blood sample, the concentration was similar regardless of the addition of the stabilizing reagent. However, the concentration of microsampler sample decreased by approximately 1/4 when the stabilizing reagent was absent (Table 5).
Previous studies have reported that acetylsalicylic acid is easily hydrolyzed into salicylic acid, which makes it difficult to quantitate its concentration.[17] Moreover, acetylsalicylic acid was known be converted to salicylic acid when air dried or left in the air, resulting in poor stability. In this study, before applying VAMS device to clinical practice, we studied to compare the concentration of whole blood samples and microsampler samples with the presence or absence of stabilizing reagents.
When acetylsalicylic acid was analyzed by whole blood sample treatment, there was no difference in the concentration of acetylsalicylic acid both regardless of the use of the stabilizing reagent. The reason for this seems to be that the analysis was performed immediately after blood collection and pretreatment. The concentration decreased when the stabilizing reagent was not added to the microsampler sample. This indicates that the stabilizing reagent is required to apply VAMS technology as a new blood sampling method. This study may be helpful for clinical use of the VAMS technology.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2017R1C1B2011095).
References
1. Denniff P, Parry S, Dopson W, Spooner N. Quantitative bioanalysis of paracetamol in rats using volumetric absorptive microsampling (VAMS). J Pharm Biomed Anal. 2015; 108:61–69. DOI: 10.1016/j.jpba.2015.01.052. PMID: 25710904.

2. De Kesel PM, Lambert WE, Stove CP. Does volumetric absorptive microsampling eliminate the hematocrit bias for caffeine and paraxanthine in dried blood samples? A comparative study. Anal Chim Acta. 2015; 881:65–73. DOI: 10.1016/j.aca.2015.04.056. PMID: 26041521.

3. Denniff P, Spooner N. Volumetric absorptive microsampling: a dried sample collection technique for quantitative bioanalysis. Anal Chem. 2014; 86:8489–8495. DOI: 10.1021/ac5022562. PMID: 25058158.

4. Kip AE, Kiers KC, Rosing H, Schellens JHM, Beijnen JH, Dorlo TPC. Volumetric absorptive microsampling (VAMS) as an alternative to conventional dried blood spots in the quantification of miltefosine in dried blood samples. J Pharm Biomed Anal. 2017; 135:160–166. DOI: 10.1016/j.jpba.2016.12.012. PMID: 28033553.

5. Mano Y, Kita K, Kusano K. Hematocrit-independent recovery is a key for bioanalysis using volumetric absorptive microsampling devices, Mitra. Bioanalysis. 2015; 7:1821–1829. DOI: 10.4155/bio.15.111. PMID: 26295984.
6. Buchanan MR, Rischke JA, Hirsh J. Aspirin inhibits platelet function independent of the acetylation of cyclo-oxygenase. Thromb Res. 1982; 25:363–373. PMID: 7071811.
7. Lemos Silva R, Carvalho de Sousa J, Calisto C, Braz Nogueira JM, Ravara L. Oral anticoagulant therapy. Fundamentals, clinical practice and recommendations. Rev Port Cardiol. 2007; 26:769–788. PMID: 17939586.
8. Jeong KH, Kim JY, Choi YS, Lee MY, Kim SY. Influence of aspirin on pilocarpine-induced epilepsy in mice. Korean J Physiol Pharmacol. 2013; 17:15–21. DOI: 10.4196/kjpp.2013.17.1.15. PMID: 23439794.

9. Singh GB, Leach GD, Atal CK. Antiinflammatory actions of methyl- and phenyl-3-methoxy-4-hydroxy styryl ketones. Arzneimittelforschung. 1987; 37:435–440. PMID: 3496890.
10. Ali MA, Routh JI. The protein binding of acetylsalicylic acid and salicylic acid. Clin Chem. 1969; 15:1027–1038. PMID: 5357059.

11. Ibrahim H, Boyer A, Bouajila J, Couderc F, Nepveu F. Determination of non-steroidal anti-inflammatory drugs in pharmaceuticals and human serum by dual-mode gradient HPLC and fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 857:59–66.

12. Feldman M, Shewmake K, Cryer B. Time course inhibition of gastric and platelet COX activity by acetylsalicylic acid in humans. Am J Physiol Gastrointest Liver Physiol. 2000; 279:G1113–G1120. PMID: 11053009.

13. Castillo-García ML, Aguilar-Caballos MP, Gómez-Hens A. Determination of acetylsalicylic acid and its major metabolites in bovine urine using ultra performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2015; 985:85–90. DOI: 10.1016/j.jchromb.2015.01.026.

14. Fogel J, Epstein P, Chen P. Simultaneous high-performance liquid chromatography assay of acetylsalicylic acid and salicylic acid in film-coated aspirin tablets. J Chromatogr. 1984; 317:507–511. PMID: 6530452.

15. O'Kruk RJ, Adams MA, Philp RB. Rapid and sensitive determination of acetylsalicylic acid and its metabolites using reversed-phase high-performance liquid chromatography. J Chromatogr. 1984; 310:343–352. PMID: 6511852.
16. Zaugg S, Zhang X, Sweedler J, Thormann W. Determination of salicylate, gentisic acid and salicyluric acid in human urine by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr B Biomed Sci Appl. 2001; 752:17–31. PMID: 11254191.

17. Gu N, Kim BH, Chung YJ, Lim KS, Seo HB, Yim DS, et al. Influence of Simvastatin on Pharmacokinetics/Pharmacodynamics of Aspirin after Oral Co-administration in Healthy Volunteers. J Korean Soc Clin Pharmacol Ther. 2011; 19:73–83.

18. Nagelschmitz J, Blunck M, Kraetzschmar J, Ludwig M, Wensing G, Hohlfeld T. Pharmacokinetics and pharmacodynamics of acetylsalicylic acid after intravenous and oral administration to healthy volunteers. Clin Pharmacol. 2014; 6:51–59. DOI: 10.2147/CPAA.S47895. PMID: 24672263.

19. Bae SK, Seo KA, Jung EJ, Kim HS, Yeo CW, Shon JH, et al. Determination of acetylsalicylic acid and its major metabolite, salicylic acid, in human plasma using liquid chromatography-tandem mass spectrometry: application to pharmacokinetic study of Astrix in Korean healthy volunteers. Biomed Chromatogr. 2008; 22:590–595. DOI: 10.1002/bmc.973. PMID: 18254152.

20. Halder D, Dan S, Biswas E, Sarkar P, Halder UC, Pal TK. A Rapid LCESI-MS/MS Method for the Quantitation of Salicylic Acid, an Active Metabolite of Acetylsalicylic Acid. Appl Clin Res Clin Trials Regul Aff. 2015; 2:90–102.
Figure 1
Production mass spectra and the pattern of fragmentation of acetylsalicylic acid (A) and hydrochlorothiazide as an internal standard (B).
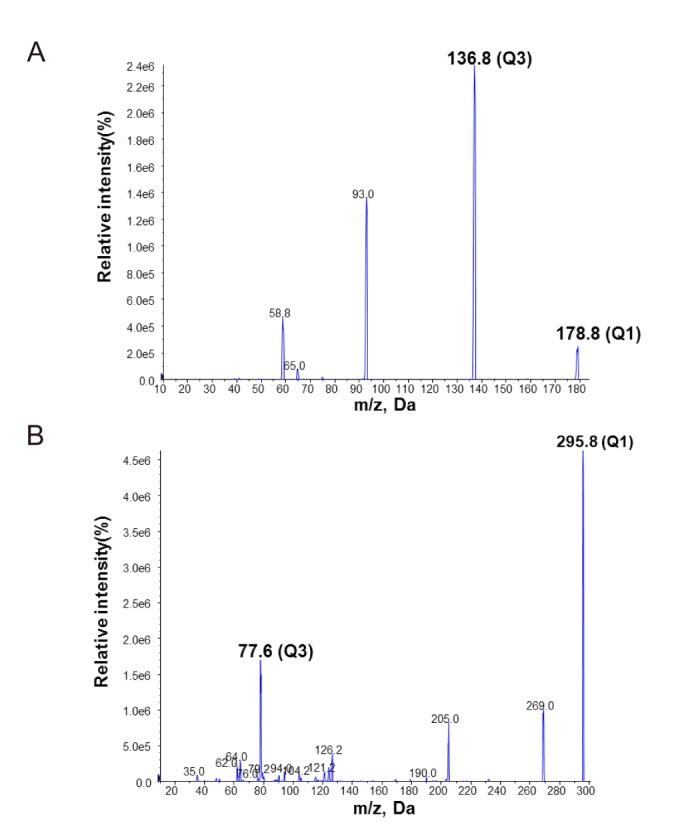
Figure 2
(A) Comparison of pipetted and absorbed volume on VAMS devices for blood; (B) absorbed volumes on VAMS devices for blood after several exposure times; (C) difference in blood absorption by VAMS sample according to the operators.
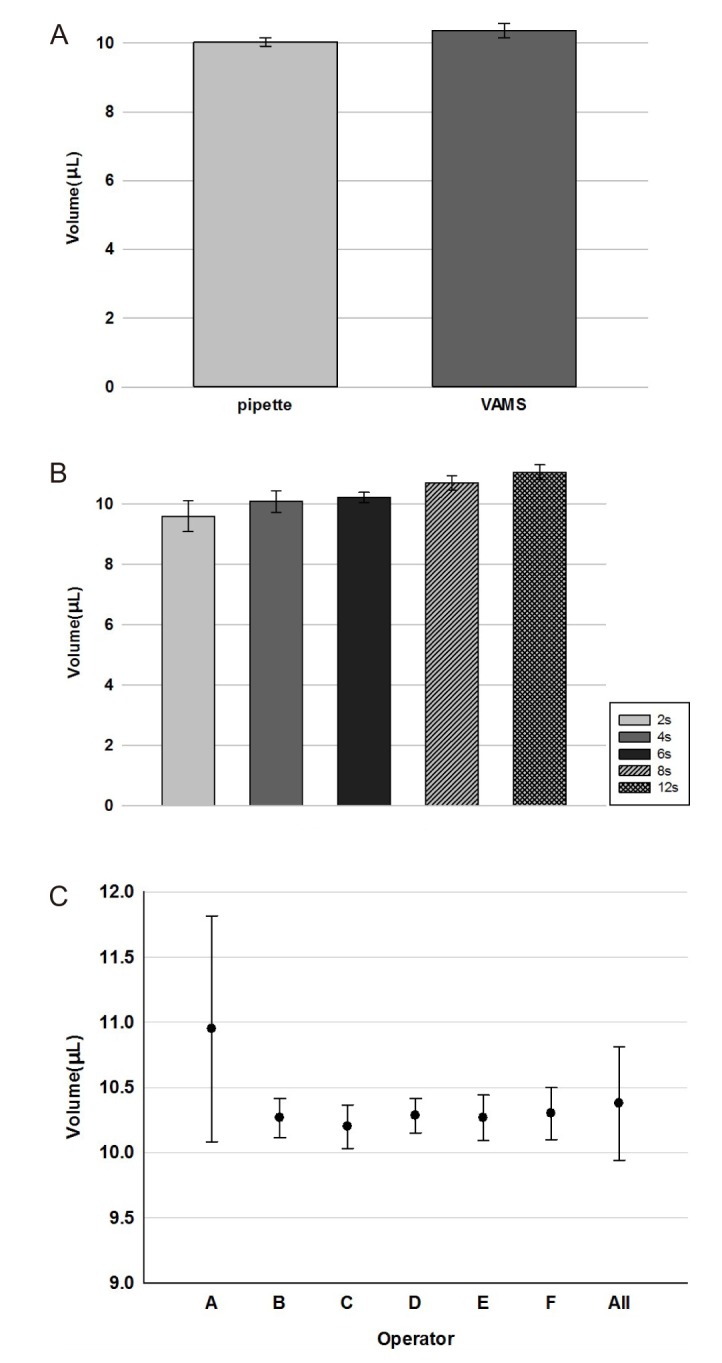
Figure 3
Representative LC-MS/MS chromatogram of double blank (A), an acetylsalicylic acid (B) and hydrochlorothiazide as an internal standard (C) at the LLOQ (40 ng/mL).
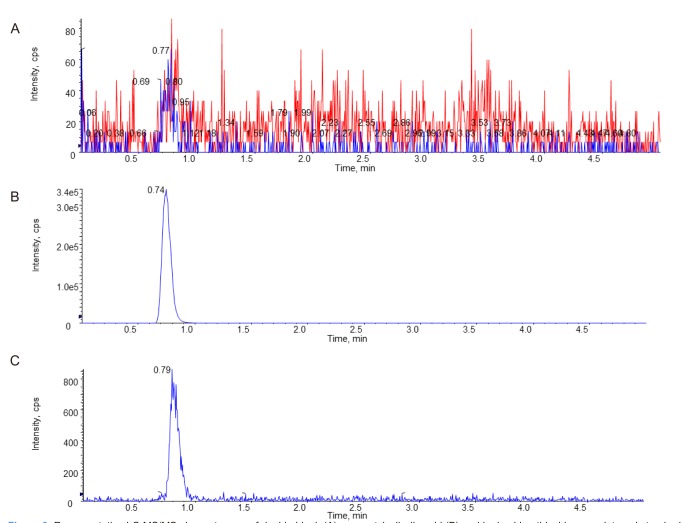
Table 1
Precision and accuracy for the determination of acetylsalicylic acid standard calibration in whole blood
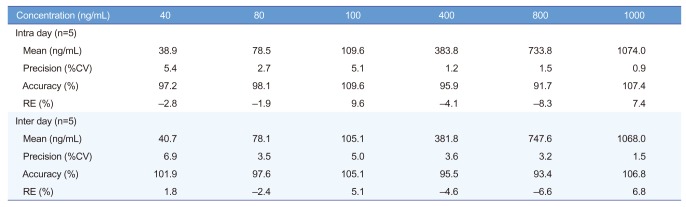
Table 2
Precision and accuracy for the determination of acetylsalicylic acid QC sample in whole blood
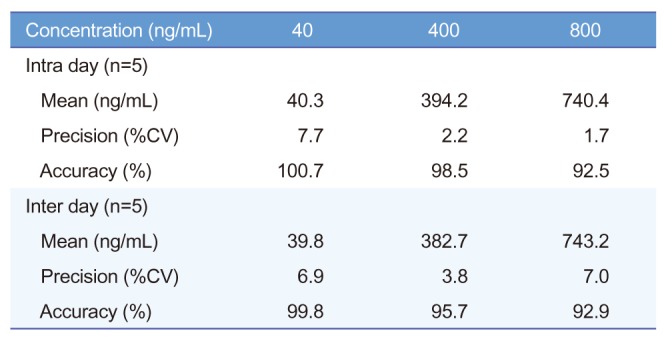
Table 3
Recovery for the determination of the acetylsalicylic acid in whole blood (n=5)
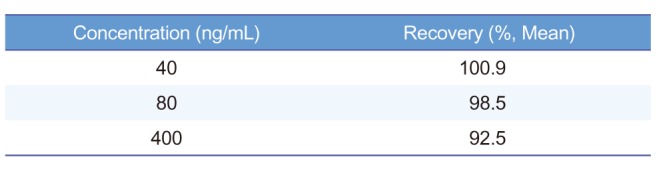
| Concentration (ng/mL) | Recovery (%, Mean) |
|---|---|
| 40 | 100.9 |
| 80 | 98.5 |
| 400 | 92.5 |
Table 4
Stability data for acetylsalicylic acid in human microsampler samples (n=3)
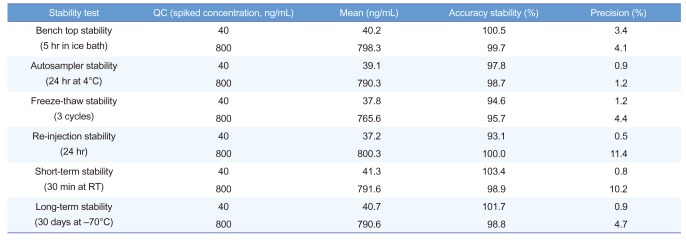
Table 5
Calculated concentrations of acetylsalicylic acid for whole blood sample and microsampler sample (n=3)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download