Various factors have been proposed as contributors to PFOA development after ACL reconstruction, including concomitant damage to articular cartilage, meniscal resection, quadriceps muscle weakness, follow-up duration, age, sex, body mass index, and limitation of knee range of motion (ROM).
45) Among them, altered knee kinematics, quadriceps weakness, and limited ROM influencing patellofemoral biomechanics have been known as risk factors resulting from inadequate postoperative rehabilitation.
46) However, the clinically important factors in the development of PFOA are still not fully known. Moreover, the prevalence and clinical effects of PFOA after ACL reconstruction with HT autograft have been rarely reported. Therefore, there is a need to investigate the prevalence of PFOA and contributors to PFOA after ACL reconstruction with HT autograft, and it could help determine proper rehabilitation strategies and lead to improved physical functions.
The present study aimed to evaluate the prevalence of PFOA and identify the factors that affect PFOA development after single-bundle ACL reconstruction with hamstring autograft. We hypothesized that quadriceps weakness and age would be significant predictors of the development of PFOA, and detecting these factors could be helpful in establishment of a rehabilitation strategy to focus on the modifiable factors such as quadriceps weakness.
METHODS
We reviewed a cohort of 324 consecutive patients who underwent primary ACL reconstruction performed by an experienced single surgeon (JGK) between January 2010 and June 2013. To be included in this study, subjects had to meet the following criteria: the patients (1) who underwent single-bundle ACL reconstruction using hamstring autograft; (2) who were available for follow-up for a minimum of 36 months after the surgery; (3) who underwent second-look arthroscopy at 24 months after the surgery; (4) who did not have any pre-existing clinical complaints of anterior knee pain; and (5) who had compliance with undergoing all required tests and questionnaires. Exclusion criteria were multiple ligament injuries, concomitant fractures, contralateral injuries, revision surgeries including meniscal procedure, and infection. The patients with a history of cartilage procedure such as microfracture or mosaicplasty were also excluded. Of the 324 patients who were originally identified, 92 who met the above criteria were enrolled. The current study was approved by the Institutional Review Board of Inje University Seoul Paik Hospital (IRB No. IIT-2015-011), and all included patients provided written informed consent.
Surgical Technique
Arthroscopic anatomic ACL reconstruction with a modified transtibial technique using hamstring autograft was conducted at least 3–4 weeks after injury by an experienced single surgeon (JGK) when knee ROM was fully obtained.
7) After making tunnels and graft passage, femoral fixation was achieved using the XO Button (ConMed, Largo, FL, USA) and the Bio-Cross Pin (RIGIDFIX; Depuy, Raynham, MA, USA). Tibial fixation was performed with a bioabsorbable interference screw (Matryx, ConMed), and additional cortical screw and washer were used for tibial fixation. For accelerated rehabilitation, this dual fixation was performed.
Postoperative Rehabilitation
Patients were permitted to bear weight with an ACL support brace (DonJoy Legend; DJO Global, Vista, CA, USA) and to start isometric quadriceps exercise and ROM exercise as tolerated immediately after ACL reconstruction based on our accelerated rehabilitation program.
8) The brace was locked in extension for ambulation and unlocked for ROM exercise. When the meniscus was repaired, partial weight bearing with crutches was performed for 6 weeks. Closed kinetic chain exercises were started at 3 weeks and open kinetic exercises and perturbation training program were started 6 weeks postoperatively. But, hamstring curl exercise was permitted from 3 months after ACL reconstruction. Quadriceps open kinetic exercises using a leg extension machine was started at 6 weeks after ACL reconstruction within 90°–45° and full extension was permitted at 3 months after the surgery. After the course of home-based rehabilitation, running and competitive sports were targeted at 3 and 9 months after ACL reconstruction, respectively.
Clinical Assessments
Clinical and functional performance tests (FPTs) were performed before the surgery and at 3, 6, 9, and 12 months or more after the surgery and were usually completed at 36 months of follow-up. Subjective knee function assessments included Lysholm score, International Knee Documentation Committee subjective knee score, and Tegner activity scale. Objective assessments included KT-2000 arthrometer (MEDmetric, San Diego, CA, USA) test, ROM measurement, manual pivot shift test, FPTs, and isokinetic muscle strength test using a Biodex System III dynamometer (Biodex, Shirley, NY, USA). Standard isokinetic muscle strength was assessed in a sitting position at angular velocities of 60°/sec and 180°/sec. The peak torque (the maximum value during four repetitions) of flexor and extensor muscles was assessed, and the value of the involved side was compared with that of the uninvolved side. The hamstring to quadriceps ratio of the involved side was calculated at 60°/sec and 180°/sec.
On FPTs, the patients were asked to perform three practice trials for each type of the tests after warming-up. For the single leg hop for distance test reported by Noyes et al.
9) as a unilateral test, each leg was tested individually and the highest values were recorded. Limb symmetry index (LSI, %) was calculated by dividing involved limb data by uninvolved limb data and then multiplying by 100.
9) The cocontraction test, Shuttle-run test, and Carioca test were performed according to previous protocols,
10) and the shortest time was recorded.
Arthroscopic Assessments
Arthroscopic cartilage evaluation allows for more detailed description of the depth and extent of arthritic lesions and detection of subtle changes such as cartilage softening, fibrillation, and tangential flaking.
11) Cartilage lesion grading was performed by a single senior surgeon (JGK) using the modified Outerbridge classification
12) at both the beginning and end of the surgery, and the grading was based on the most severe cartilage damage. Grade 2 and above cartilage lesion was defined by the Outerbridge classification as any damage at each compartment (patellofemoral, medial tibiofemoral, and lateral tibiofemoral).
4) Concomitant meniscal injury was also evaluated and defined as a meniscal tear requiring surgery such as meniscectomy and repair.
13) In the present study, meniscectomy was defined as meniscal resection of > 33% of the whole meniscus.
Second-look arthroscopy with hardware removal was performed usually at 24 months after the primary ACL reconstruction, especially in patients who had persistent pain, wanted to remove the irritable materials, or voluntarily wanted to confirm the status of the reconstructed ACL. Most patients wanted to a second-look arthroscopy with hardware removal.
Statistical Analysis
Statistical analysis was done using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA). Pearson chi-square test, Student t-test, or the Mann-Whitney U-test were performed to compare results between the PFOA and the no PFOA groups, depending on the parametric or nonparametric data distribution. A univariable logistic regression analysis was implemented to determine significant factors for developing PFOA. Then, a multivariable stepwise logistic regression was performed using the significant factors identified in the univariable logistic regression. The results of regression analysis were presented as odds ratio (OR) with 95% confidence interval (CI), and a p-value < 0.05 was considered significant.
Go to :

RESULTS
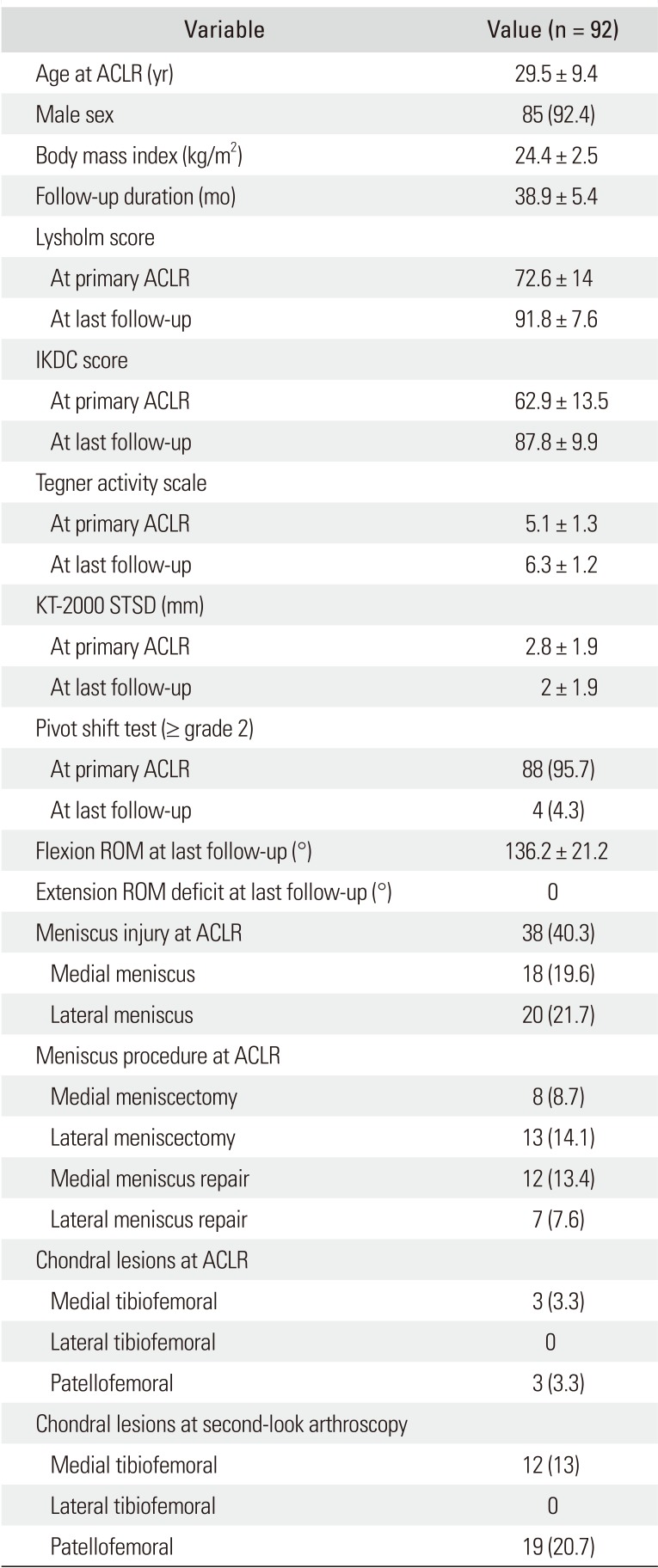
Ninety-two patients, including 85 males and 7 females, were evaluated, and the mean follow-up was 38.9 ± 5.4 months. The patient's baseline characteristics are presented in
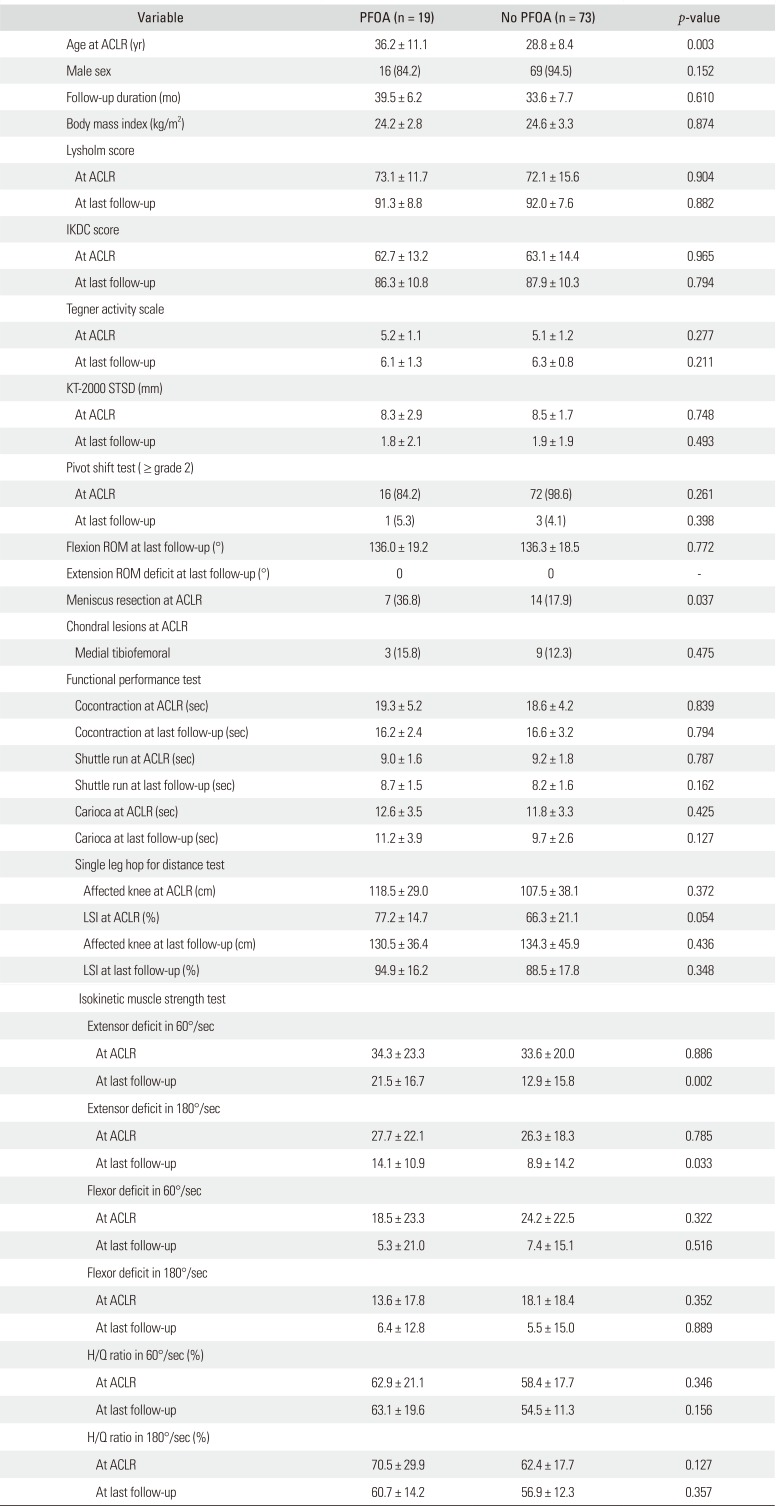
Table 1. Their mean age at ACL reconstruction was 29.5 ± 9 years. Concomitant meniscal injuries at the time of ACL reconstruction were detected in 38 patients (40.3%), including 18 medial meniscus (19.6%) and 20 lateral meniscus injuries (21.7%). Eight subjects (8.7%) had a medial meniscectomy, and 13 (14.1%) had a lateral meniscectomy; thus, total 21 patients (22.8%) underwent a concurrent meniscectomy during ACL reconstruction. PFOA above grade 2 according to the Outerbridge classification was observed in 19 patients (20.7%) at the second-look arthroscopy. Of them, three patients (3.3%) with preexisting PFOA showed progression of the cartilage lesion, and 16 (17.4%) had newly developed PFOA. The patient characteristics for those with PFOA are described in
Table 2. All patients did not have any pre-existing clinical complaints of anterior knee pain, and 19 (100%) patients with PFOA development presented with anterior knee pain, while six patients (8.2%) without PFOA development showed anterior knee pain. Three factors were significantly different (
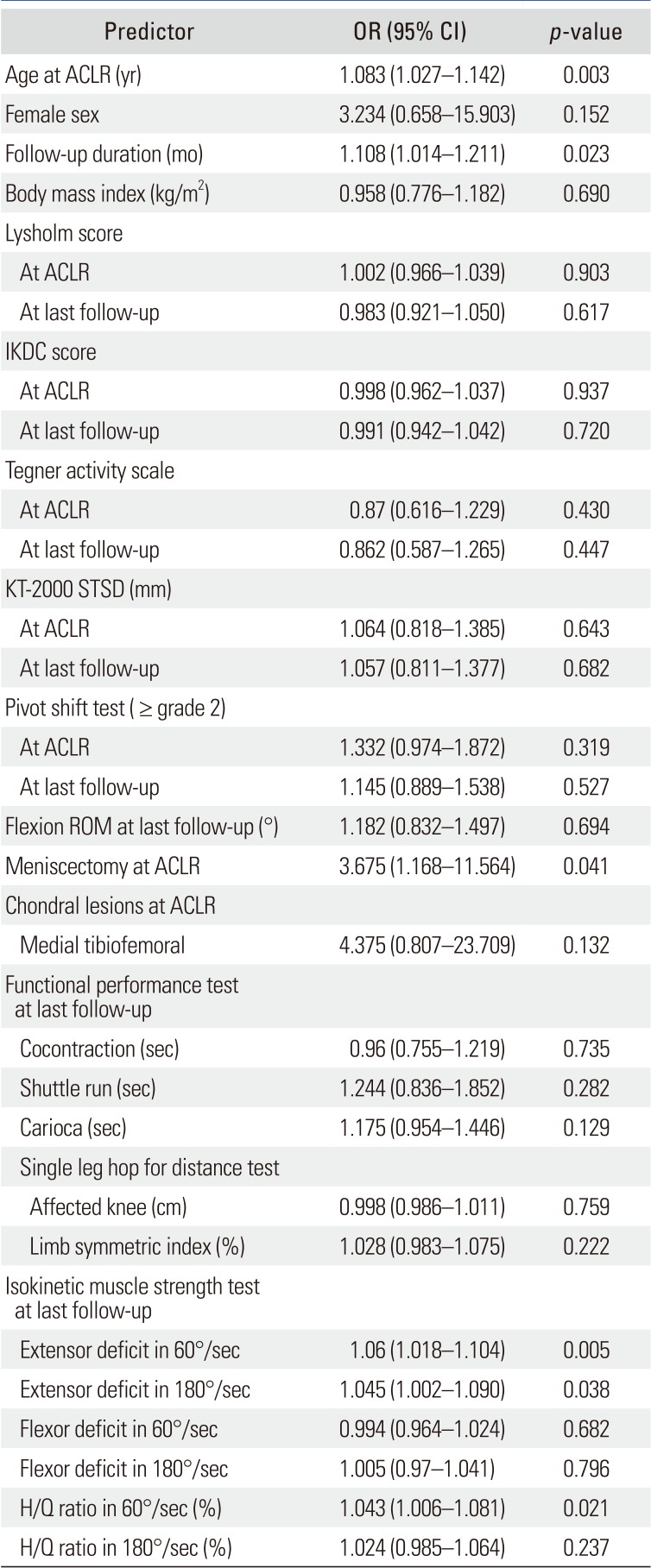
p < 0.05) between the PFOA and no PFOA groups: age at ACL reconstruction, meniscectomy at primary surgery, and isokinetic extensor deficit at the second-look arthroscopy. In the univariate logistic regression analysis (
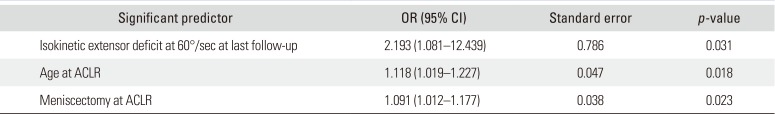
Table 3), PFOA was significantly associated with age at ACL reconstruction (OR, 1.083; 95% CI, 1.027 to 1.142), follow-up duration (OR, 1.108; 95% CI, 1.014 to 1.211), concurrent meniscectomy at ACL reconstruction (OR, 3.675; 95% CI, 1.168 to 11.564), and isokinetic extensor strength deficit at the last follow-up (60°/sec: OR, 1.06; 95% CI, 1.018 to 1.104; 180°/sec: OR, 1.045; 95% CI, 1.002 to 1.090). The results of the multivariate analysis are shown in
Table 4. The isokinetic extensor deficit at 60°/sec at the last follow-up (
p = 0.031; OR, 2.193; 95% CI, 1.081 to 12.439), age at primary ACL reconstruction (
p = 0.018; OR, 1.118; 95% CI, 1.019 to 1.227), and concurrent meniscectomy at primary surgery (
p = 0.023; OR, 0.091; 95% CI, 1.012 to 1.177) were the significant predictors of PFOA development.
Table 1
Demographics and Pre- and Postoperative Data
|
Variable |
Value (n = 92) |
|
Age at ACLR (yr) |
29.5 ± 9.4 |
|
Male sex |
85 (92.4) |
|
Body mass index (kg/m2) |
24.4 ± 2.5 |
|
Follow-up duration (mo) |
38.9 ± 5.4 |
|
Lysholm score |
|
|
At primary ACLR |
72.6 ± 14 |
|
At last follow-up |
91.8 ± 7.6 |
|
IKDC score |
|
|
At primary ACLR |
62.9 ± 13.5 |
|
At last follow-up |
87.8 ± 9.9 |
|
Tegner activity scale |
|
|
At primary ACLR |
5.1 ± 1.3 |
|
At last follow-up |
6.3 ± 1.2 |
|
KT-2000 STSD (mm) |
|
|
At primary ACLR |
2.8 ± 1.9 |
|
At last follow-up |
2 ± 1.9 |
|
Pivot shift test (≥ grade 2) |
|
|
At primary ACLR |
88 (95.7) |
|
At last follow-up |
4 (4.3) |
|
Flexion ROM at last follow-up (°) |
136.2 ± 21.2 |
|
Extension ROM deficit at last follow-up (°) |
0 |
|
Meniscus injury at ACLR |
38 (40.3) |
|
Medial meniscus |
18 (19.6) |
|
Lateral meniscus |
20 (21.7) |
|
Meniscus procedure at ACLR |
|
|
Medial meniscectomy |
8 (8.7) |
|
Lateral meniscectomy |
13 (14.1) |
|
Medial meniscus repair |
12 (13.4) |
|
Lateral meniscus repair |
7 (7.6) |
|
Chondral lesions at ACLR |
|
|
Medial tibiofemoral |
3 (3.3) |
|
Lateral tibiofemoral |
0 |
|
Patellofemoral |
3 (3.3) |
|
Chondral lesions at second-look arthroscopy |
|
|
Medial tibiofemoral |
12 (13) |
|
Lateral tibiofemoral |
0 |
|
Patellofemoral |
19 (20.7) |

Table 2
Comparison of Patient Characteristics between Groups with and without PFOA
|
Variable |
PFOA (n = 19) |
No PFOA (n = 73) |
p-value |
|
Age at ACLR (yr) |
36.2 ± 11.1 |
28.8 ± 8.4 |
0.003 |
|
Male sex |
16 (84.2) |
69 (94.5) |
0.152 |
|
Follow-up duration (mo) |
39.5 ± 6.2 |
33.6 ± 7.7 |
0.610 |
|
Body mass index (kg/m2) |
24.2 ± 2.8 |
24.6 ± 3.3 |
0.874 |
|
Lysholm score |
|
|
|
|
At ACLR |
73.1 ± 11.7 |
72.1 ± 15.6 |
0.904 |
|
At last follow-up |
91.3 ± 8.8 |
92.0 ± 7.6 |
0.882 |
|
IKDC score |
|
|
|
|
At ACLR |
62.7 ± 13.2 |
63.1 ± 14.4 |
0.965 |
|
At last follow-up |
86.3 ± 10.8 |
87.9 ± 10.3 |
0.794 |
|
Tegner activity scale |
|
|
|
|
At ACLR |
5.2 ± 1.1 |
5.1 ± 1.2 |
0.277 |
|
At last follow-up |
6.1 ± 1.3 |
6.3 ± 0.8 |
0.211 |
|
KT-2000 STSD (mm) |
|
|
|
|
At ACLR |
8.3 ± 2.9 |
8.5 ± 1.7 |
0.748 |
|
At last follow-up |
1.8 ± 2.1 |
1.9 ± 1.9 |
0.493 |
|
Pivot shift test (≥ grade 2) |
|
|
|
|
At ACLR |
16 (84.2) |
72 (98.6) |
0.261 |
|
At last follow-up |
1 (5.3) |
3 (4.1) |
0.398 |
|
Flexion ROM at last follow-up (°) |
136.0 ± 19.2 |
136.3 ± 18.5 |
0.772 |
|
Extension ROM deficit at last follow-up (°) |
0 |
0 |
- |
|
Meniscus resection at ACLR |
7 (36.8) |
14 (17.9) |
0.037 |
|
Chondral lesions at ACLR |
|
|
|
|
Medial tibiofemoral |
3 (15.8) |
9 (12.3) |
0.475 |
|
Functional performance test |
|
|
|
|
Cocontraction at ACLR (sec) |
19.3 ± 5.2 |
18.6 ± 4.2 |
0.839 |
|
Cocontraction at last follow-up (sec) |
16.2 ± 2.4 |
16.6 ± 3.2 |
0.794 |
|
Shuttle run at ACLR (sec) |
9.0 ± 1.6 |
9.2 ± 1.8 |
0.787 |
|
Shuttle run at last follow-up (sec) |
8.7 ± 1.5 |
8.2 ± 1.6 |
0.162 |
|
Carioca at ACLR (sec) |
12.6 ± 3.5 |
11.8 ± 3.3 |
0.425 |
|
Carioca at last follow-up (sec) |
11.2 ± 3.9 |
9.7 ± 2.6 |
0.127 |
|
Single leg hop for distance test |
|
|
|
|
Affected knee at ACLR (cm) |
118.5 ± 29.0 |
107.5 ± 38.1 |
0.372 |
|
LSI at ACLR (%) |
77.2 ± 14.7 |
66.3 ± 21.1 |
0.054 |
|
Affected knee at last follow-up (cm) |
130.5 ± 36.4 |
134.3 ± 45.9 |
0.436 |
|
LSI at last follow-up (%) |
94.9 ± 16.2 |
88.5 ± 17.8 |
0.348 |
|
Isokinetic muscle strength test |
|
|
|
|
Extensor deficit in 60°/sec |
|
|
|
|
At ACLR |
34.3 ± 23.3 |
33.6 ± 20.0 |
0.886 |
|
At last follow-up |
21.5 ± 16.7 |
12.9 ± 15.8 |
0.002 |
|
Extensor deficit in 180°/sec |
|
|
|
|
At ACLR |
27.7 ± 22.1 |
26.3 ± 18.3 |
0.785 |
|
At last follow-up |
14.1 ± 10.9 |
8.9 ± 14.2 |
0.033 |
|
Flexor deficit in 60°/sec |
|
|
|
|
At ACLR |
18.5 ± 23.3 |
24.2 ± 22.5 |
0.322 |
|
At last follow-up |
5.3 ± 21.0 |
7.4 ± 15.1 |
0.516 |
|
Flexor deficit in 180°/sec |
|
|
|
|
At ACLR |
13.6 ± 17.8 |
18.1 ± 18.4 |
0.352 |
|
At last follow-up |
6.4 ± 12.8 |
5.5 ± 15.0 |
0.889 |
|
H/Q ratio in 60°/sec (%) |
|
|
|
|
At ACLR |
62.9 ± 21.1 |
58.4 ± 17.7 |
0.346 |
|
At last follow-up |
63.1 ± 19.6 |
54.5 ± 11.3 |
0.156 |
|
H/Q ratio in 180°/sec (%) |
|
|
|
|
At ACLR |
70.5 ± 29.9 |
62.4 ± 17.7 |
0.127 |
|
At last follow-up |
60.7 ± 14.2 |
56.9 ± 12.3 |
0.357 |

Table 3
Univariable Regression Analysis for Predictors of Patellofemoral Osteoarthritis
|
Predictor |
OR (95% CI) |
p-value |
|
Age at ACLR (yr) |
1.083 (1.027–1.142) |
0.003 |
|
Female sex |
3.234 (0.658–15.903) |
0.152 |
|
Follow-up duration (mo) |
1.108 (1.014–1.211) |
0.023 |
|
Body mass index (kg/m2) |
0.958 (0.776–1.182) |
0.690 |
|
Lysholm score |
|
|
|
At ACLR |
1.002 (0.966–1.039) |
0.903 |
|
At last follow-up |
0.983 (0.921–1.050) |
0.617 |
|
IKDC score |
|
|
|
At ACLR |
0.998 (0.962–1.037) |
0.937 |
|
At last follow-up |
0.991 (0.942–1.042) |
0.720 |
|
Tegner activity scale |
|
|
|
At ACLR |
0.87 (0.616–1.229) |
0.430 |
|
At last follow-up |
0.862 (0.587–1.265) |
0.447 |
|
KT-2000 STSD (mm) |
|
|
|
At ACLR |
1.064 (0.818–1.385) |
0.643 |
|
At last follow-up |
1.057 (0.811–1.377) |
0.682 |
|
Pivot shift test (≥ grade 2) |
|
|
|
At ACLR |
1.332 (0.974–1.872) |
0.319 |
|
At last follow-up |
1.145 (0.889–1.538) |
0.527 |
|
Flexion ROM at last follow-up (°) |
1.182 (0.832–1.497) |
0.694 |
|
Meniscectomy at ACLR |
3.675 (1.168–11.564) |
0.041 |
|
Chondral lesions at ACLR |
|
|
|
Medial tibiofemoral |
4.375 (0.807–23.709) |
0.132 |
|
Functional performance test at last follow-up |
|
|
|
Cocontraction (sec) |
0.96 (0.755–1.219) |
0.735 |
|
Shuttle run (sec) |
1.244 (0.836–1.852) |
0.282 |
|
Carioca (sec) |
1.175 (0.954–1.446) |
0.129 |
|
Single leg hop for distance test |
|
|
|
Affected knee (cm) |
0.998 (0.986–1.011) |
0.759 |
|
Limb symmetric index (%) |
1.028 (0.983–1.075) |
0.222 |
|
Isokinetic muscle strength test at last follow-up |
|
|
|
Extensor deficit in 60°/sec |
1.06 (1.018–1.104) |
0.005 |
|
Extensor deficit in 180°/sec |
1.045 (1.002–1.090) |
0.038 |
|
Flexor deficit in 60°/sec |
0.994 (0.964–1.024) |
0.682 |
|
Flexor deficit in 180°/sec |
1.005 (0.97–1.041) |
0.796 |
|
H/Q ratio in 60°/sec (%) |
1.043 (1.006–1.081) |
0.021 |
|
H/Q ratio in 180°/sec (%) |
1.024 (0.985–1.064) |
0.237 |

Table 4
Predictors Associated with Patellofemoral Osteoarthritis in Stepwise Multivariable Logistic Regression Analysis
|
Significant predictor |
OR (95% CI) |
Standard error |
p-value |
|
Isokinetic extensor deficit at 60°/sec at last follow-up |
2.193 (1.081–12.439) |
0.786 |
0.031 |
|
Age at ACLR |
1.118 (1.019–1.227) |
0.047 |
0.018 |
|
Meniscectomy at ACLR |
1.091 (1.012–1.177) |
0.038 |
0.023 |

Go to :

DISCUSSION
The principal finding of the current study was that the significant predictors of PFOA after ACL reconstruction with hamstring autograft were (1) quadriceps muscle weakness at last follow-up, (2) increased age at primary surgery, and (3) concurrent meniscectomy at primary surgery. Among them, quadriceps strengthening was a modifiable factor. In addition, the prevalence of PFOA development after ACL reconstruction was 20.7% (3.3% pre-existing PFOA and 17.4% newly developed PFOA) at a mean follow-up of 38.9 months.
The development of chondral lesion after ACL reconstruction is the results of biochemical and biomechanical changes, and chondral lesions are mainly observed in the patellofemoral joint.
4) The median prevalence of PFOA at 10 to 15 years after ACL reconstruction is almost 50%.
14) If we evaluate the association between PFOA and predictors including muscle strength after ACL reconstruction at short-term follow-up using second-look arthroscopy, additional rehabilitation programs to correct modifiable factors for risk of PFOA could be performed to prevent structural cartilage changes in the longer term.
Culvenor et al.
14) conducted a literature review about the prevalence of PFOA after ACL reconstruction and risk factors of PFOA and concluded as follows: (1) there was a high prevalence of PFOA after ACL reconstruction as frequently as tibiofemoral osteoarthritis, irrespective of graft type; (2) several mechanisms existed for PFOA after ACL reconstruction; (3) it would be important to address factors contributing to PFOA development during rehabilitation. The proposed predictors of PFOA development which they evaluated were concomitant damage to the cartilage and meniscus, altered knee biomechanics, decreased quadriceps strength, and inflammation. Culvenor et al.
5) reported that 30% had patellofemoral joint problem after ACL reconstruction with hamstring autograft at 12 months of follow-up and older age at ACL reconstruction was the only predictor of postoperative patellofemoral pain, and they emphasized postoperative rehabilitation in patients with ≥ 27 years of age. In our study, the PFOA above grade 2 according to the Outerbridge classification was found in 20.7% at a mean follow-up of 38.9 months, and of them, 3.3% had pre-existing PFOA which showed progression of cartilage lesion, and 17.4% had newly developed PFOA. The contributors to a greater chance of PFOA development after ACL reconstruction with hamstring autograft in our study were decreased quadriceps strength at last follow-up, increased age and concurrent meniscectomy at primary surgery, which is congruent with previous studies.
514)
Quadriceps weakness is a common finding in patients who underwent ACL reconstruction, in whom quadriceps strength deficits have been reported at rates of 2%–20%.
15) Urbach et al.
16) reported that unilateral ACL rupture could lead to inhibition of the quadriceps muscle in both the involved and uninvolved limbs, showing voluntary-activation deficit and true muscle weakness. Decreased quadriceps strength is related to peripheral changes in the muscle–tendon units of the quadriceps muscle.
17) These peripheral changes include chronic atrophy, changes in compliance of the series of elastic components in the muscle–tendon units, and alterations in the architectural structure and composition (fiber type) of the quadriceps muscle.
18) A recent systematic review proved that the patients who underwent ACL reconstruction showed decreased knee extensor moments and flexion angles in performing tasks such as walking, running, and single-leg landing.
19) Culvenor et al.
20) reported that the participants with early PFOA after ACL reconstruction with hamstring autograft at 12–24 months of follow-up exhibited smaller peak knee flexion angles and extensor moments, and greater knee internal rotation excursion during hop task, compared to those without PFOA using three-dimensional motion analysis. They suggested that lower peak knee flexion angles and extensor moments might reflect quadriceps weakness; hence, rehabilitation strategies focusing neuromuscular deficits associated these abnormal post-ACL reconstruction biomechanics could increase the longevity of patellofemoral cartilage. Altered kinematics and kinetics, such as decreased knee flexion angles and moments, and increased knee internal rotation excursion during high-demand tasks resulted from quadriceps weakness, can increase the risk of early PFOA progression by limiting the contact area over which large patellofemoral loads can be distributed and create more localized areas of contact stress.
20) Amin et al.
21) performed a prospective study with 265 subjects to analyze the 30-month natural history of knee osteoarthritis. They concluded that greater quadriceps strength was protective against cartilage lesion at the lateral patellofemoral joint and there was no association between quadriceps strength and cartilage lesion at the tibiofemoral joint. Wang et al.
22) reported that greater than 80% recovery of quadriceps strength after ACL reconstruction using hamstring autograft in 42 patients was associated with less severe patellofemoral joint cartilage damaged at an average of 24.1 months. Our results support previous reports of a significant association between quadriceps strength weakness and PFOA development after ACL reconstruction (
p = 0.031; OR, 2.193; 95% CI, 1.081 to 12.439) at the last follow-up. Hence, our suggestion is that isokinetic knee extensor strength demonstrating a LSI lower than 20% is an appropriate result of adequate postoperative muscle rehabilitation. Brasileiro et al.
23) demonstrated that eccentric training twice a week proved to be a potent resource for the quadriceps recovery, both morphologically and functionally. Quadriceps open kinetic exercises using a leg extension machine was started at 6 weeks after ACL reconstruction within 90°–45° and full extension was permitted at 3 months after the surgery. Although our patients performed rehabilitation program at home by themselves, they visited our clinic at 3, 6, 9, 12 months to evaluate their isokinetic muscle strength and functional performance.
Increased age at primary surgery was a significant contributor to PFOA development, with the PFOA group being older than the no PFOA group (
p = 0.003), and middle-aged patients (≥ 40 years) had increased risk of PFOA development compared with younger patients (< 40 years) (OR, 12.407; 95% CI, 3.633 to 42.374;
p < 0.001) in our study. Even though ACL reconstruction usually has resulted in good outcomes for middle-aged patients, sometimes the outcomes from ACL reconstruction have not been different subjectively or objectively between patients ≥ 40 years and those < 40 years.
24) Despite the overall favorable outcomes, radiographic osteoarthritis including PFOA could worsen in middle-aged patients.
25) Culvenor et al.
5) evaluated the patellofemoral pain following HT ACL reconstruction in 110 patients, and they showed that the patients ≥ 27 years of age at ACL reconstruction were 2.6 times more likely to have patellofemoral pain compared to < 27 years at 12 months after the surgery. They speculated that the higher rates of patellofemoral pain might be a result of ongoing patellofemoral cartilage thinning known to develop in older people during the first 2 years after the ACL reconstruction based on previous research.
26) Musculoskeletal aging affects joint function and tissue components due to sarcopenia, loss of proprioception and balance, increased joint laxity, increased catabolic activity of articular cartilage, and tendency to become brittle of cartilage structures.
27) Altered patellofemoral biomechanics, decreased quadriceps strength, and increased focal loading could result in the imbalance in the underlying age-related tissue homeostasis exceeding the biological-mechanical threshold of cartilage, which could cause the patellofemoral cartilage to become thinner and less elastic and stimulate the highly innervated subchondral bone, ultimately leading to patellofemoral pain and progressive cartilage loss (PFOA).
28)
Concurrent meniscectomy at primary surgery was a significant predictor of PFOA development (OR, 0.091; 95% CI, 1.012 to 1.177;
p = 0.023). In the present study, concomitant meniscal injuries at the time of ACL reconstruction were found in 40.3%, including 19.6% of medial meniscus and 21.7% of lateral meniscus injuries. Among them, 8.7% got a medial meniscectomy, and 14.1% got a lateral meniscectomy; thus, total 22.8% underwent a concurrent meniscectomy during primary surgery. The relationship between meniscectomy and the development of PFOA has been reported in several studies. Englund and Lohmander
29) showed that the ORs for PFOA after medial and lateral meniscectomy without ACL injury after 20 years were 2.6 (95% CI, 1.1 to 6.6) and 5.3 (95% CI, 1.9 to 15.0), respectively. Keays et al.
30) reported meniscectomy (r = 0.45), chondral damage (r = 0.75), and age at surgery (r = 0.65) as predictors of PFOA after ACL reconstruction or close predictors at a 6-year follow-up. These findings might have resulted from the decreased contact area and increased contact stress focused within the tibiofemoral joint which would affect gait and patellofemoral joint biomechanics at the PF joint.
31) Wang et al.
32) reported that partial medial meniscectomy (< 33% of medial meniscus resection) had more prevalent PFOA (OR, 13.76; 95% CI, 1.52 to 124.80) and time from partial medial meniscectomy was positively associated with PFOA (OR, 1.04; 95% CI, 1.01 to 1.07) than medial tibiofemoral osteoarthritis. They speculated that when coupled with quadriceps weakness seen in medial meniscectomy, a varus alignment might increase the stress on the medial patellofemoral joint, consequentially increasing the risk of PFOA development. Further research is needed to identify the biomechanical effects of medial and lateral meniscectomy on the patellofemoral joint mechanics and PFOA.
Clinically, our results are emphasizing the importance of quadriceps strengthening after ACL reconstruction, especially in the older patients and meniscectomy to minimize patellofemoral joint force and stress during rehabilitation and to decrease the risk of development of PFOA. Quadriceps strengthening is an achievable target during rehabilitation and totally depends on the efforts of the clinician and patient. Again, the prevalence of PFOA after ACL reconstruction with hamstring autograft is relatively high, so the greater focus on early detection of high risk factors for PFOA progression and strategies such as optimizing quadriceps exercise and neuromuscular control are warranted.
The present study has some limitations. First, there would be a selection bias as we only included the patients who were participating in our rehabilitation protocol and clinical tests which involved high-level activities for isokinetic muscle strength tests and FPTs. Second, the study had a relatively short-term follow-up. The follow-up duration might be a critical risk factor for the development of PFOA, so the prevalence could have been lower than the incidence observed in a long-term follow-up. Third, this retrospective study lacks comparison with a cohort of patients without ACL reconstruction in terms of PFOA grades. Finally, the location and area of PFOA were not described in detail. However, we could determine the cartilage lesions' depth and extent and detected early stage subtle arthritic changes, such as cartilage softening, fibrillation, and tangential flaking through second-look arthroscopy. As there has been no consensus yet on how to combine and quantify the cartilage lesions of the patella and trochlear groove, further research based on comprehensive evaluation is needed.
In conclusion, significant predictors of PFOA after ACL reconstruction with hamstring autograft were decreased quadriceps strength at last follow-up, increased age, and concurrent meniscectomy at primary surgery. Quadriceps weakness as a modifiable factor should be considered in the establishment of a rehabilitation strategy to prevent PFOA after ACL reconstruction, especially in older age.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download