Abstract
Background
Bone mineral density (BMD) is the indicator of bone quality in at-risk individuals. Along with the fracture risk assessment tool (FRAX), a quick assessment of BMD from routine radiographs may be useful in the case of lacking X-ray absorptiometry data. This study aimed to investigate the correlation of cortical thickness index (CTI) and canal flare index (CFI) with BMD and FRAX and to evaluate their ability to predict femoral neck BMD (nBMD) and FRAX in the general elderly population.
Methods
A total of 560 volunteers (age ≥ 50 years) who underwent hip-spine X-ray, BMD scanning and FRAX calculation were retrospectively reviewed. CTI and CFI were measured on anteroposterior radiographs and analyzed for their correlation with BMD and FRAX and for their ability to predict nBMD. The ability of CTI to predict osteoporosis status (OPS) and fracture risk status (FRS) was also investigated and the threshold values were calculated. All the analyses were performed separately on male and female subjects.
Results
Significant differences in CTI, CFI, nBMD and FRAX between males and females were observed. CTI and CFI demonstrated significant positive correlation with nBMD and FRAX (all p < 0.001) in both males and females. CTI, height, and weight significantly predicted nBMD. CTI statistically predicted OPS and FRS, and the values of 0.56 and 0.62 were computed as CTI thresholds for males and females, respectively.
Osteoporosis is a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture.1) One in two women and one in five men over 50 years have an osteoporosis-related fracture in their lifetime.2) Albeit not the most common fracture, hip fracture is the most severe complication of osteoporosis.34)
Clinically, osteoporosis is estimated by assessing bone mineral density (BMD) using dual energy X-ray absorptiometry (DXA), as proposed by the World Health Organization (WHO). Internationally, the proximal hip and lumbar spine are the two most common regions for BMD measurement; however, the whole hip is a reliable site for measurement especially in people with severe lumbar osteoarthritis, which may result in a false increase in lumbar BMD.5) A fracture risk assessment tool (FRAX) for 10-year probability was also proposed by the WHO to enhance the predictive value of BMD and fracture risk.
Although DXA is the most useful BMD measurement, it is not always available and not routinely ordered, and orthopedists often lack DXA results at the time of examination. Within the Asia-Pacific area, while developed countries have 12–24 DXA machines per million, the developing countries, in contrast, are severely under-resourced with far less than one machine per million population.6) In addition, in some developed countries, the fact that DXA is not reimbursed proposes a barrier to the identification of people with osteoporosis. The FRAX may become a great help in this situation; however, there are also countries in which the surrogate FRAX models are not available yet. These are the reasons why we try to look for a supportive parameter for preliminarily estimating BMD in the case of lack of DXA results. This can be not only beneficial for the developing countries' physicians who do not have access to DXA but also sometimes helpful for orthopedists in developed countries while waiting for the DXA results.
In the modern medical practice, almost all patients with hip-related disorders who present to the orthopedists undergo hip X-ray routinely and DXA scan rarely. Hence, X-ray is a potential supportive radiographic tool in BMD evaluation and fragility fracture risk estimation when DXA data are not available. The most commonly used radiographic parameter to assess bone status is the cortical thickness index (CTI). The canal flare index (CFI), which is used to classify the medullary canal for femoral component selection in total hip arthroplasty, is also reported to be associated with aging and osteoporosis.78)
Although a significant correlation of CTI and CFI with BMD has been confirmed in several publications, not many have studied these parameters in relatively large samples representing the general population of a specific region rather than the subjects visiting the hospital. Moreover, most of the previous papers studied these correlations without the separate analysis of males and females. The authors of this study suspected that, regarding osteoporosis, there would be significant sex-related dissimilarities in the data.
The purpose of this study was (1) to separately investigate males and females for correlation analysis between the radiographic parameters (CTI and CFI) and femoral neck BMD (nBMD) and FRAX in a general elderly population; and (2) to evaluate the ability of CTI and CFI to predict BMD and FRAX.
This was a retrospective analysis of data from a cross-sectional study of 603 volunteers aged 50 and older in a town in Japan, which investigated musculoskeletal function and quality of life. A total of 560 volunteers met the inclusion criteria, which included aged 50 and older and no history of congenital hip-related pathologies. Exclusion criteria were lack of any information needed for analysis. The study was approved by the Institutional Review Board of Toei Hospital (IRB No. 201201) where the investigation was performed. All subjects were volunteers. They were carefully explained about the study.
Demographic characteristics, risk factors for FRAX calculation, hip and spine radiographs, and BMD data were obtained at the local hospital and were retrospectively reviewed. Demographic characteristics included sex, age, height and weight. Risk factors, according to the FRAX tool, included previous fractures, parent's fractured hip, current smoking status, glucocorticoids, rheumatoid arthritis, secondary osteoporosis and alcohol consumption 3 or more units/day. The radiographs were standard anteroposterior hip radiographs. BMD evaluation was performed on the left proximal femur by using a DXA system (Discovery QDR; Hologic, Marlborough, MA, USA).
Digital radiographic data were imported into medical image viewer software (OsiriX, Pixmeo, Bernex, Switzerland). The CTI and CFI of all 560 subjects were measured by a hip surgeon (BNTN) blinded to the BMD and FRAX results (Fig. 1). A set of 59 random radiographs was selected for the calculation of intra- and interobserver reliability. In addition to the hip surgeon, three other observers with different levels of clinical training were chosen to avoid the impact of experience on the measurements. These observers included a senior hip surgeon (HH), an orthopaedic graduate, and a medical technician. All four observers measured the set twice in random order with an interval of 3 months. They were all blinded to each other's findings as well as to the BMD results.
CTI was defined as the ratio of the femoral diaphyseal diameter (outer diameter [Do]) minus the intramedullary canal diameter (inner diameter [Di]) to the femoral diaphyseal diameter. These diameters were measured 10 cm below the midpoint of the lesser trochanter, as described by Dorr et al.9) Noble et al.10) described CFI as a parameter for morphologically classifying the proximal femur. It was defined as the ratio of the intracortical width of the femur at a point 20 mm proximal to the lesser trochanter (canal width) and at the canal isthmus. For consistent measurement, we identified the canal isthmus at 10 cm below the mid lesser trochanter, as defined by Yeung et al.11)
The left femur BMD of all 560 subjects was measured by using DXA at four regions: the greater trochanter, intertrochanteric region, femoral neck, and Ward's triangle. The nBMD results were used for the purpose of analysis. The FRAX was calculated from the collected risk factor information and nBMD using the official FRAX tool provided.
Based on the Japanese Society for Bone and Mineral Research (JSBMR) 2012 diagnostic criteria for primary osteoporosis, BMD was compared to the Japanese Young Adult Mean (YAM) proximal femur BMD and converted into a percentage of the YAM (% YAM).12) Subjects who did not have previous femur or vertebral fractures were categorized as osteoporosis (< 70% YAM), low bone mass (70%–80% YAM) or normal (≥ 80% YAM), which were finally reclassified into normal (≥ 80% YAM) or high-risk (< 80% YAM) osteoporosis status (OPS). The major osteoporotic fracture risk in the FRAX results were also used to categorize all 560 subjects as low-risk (< 15%) and high-risk (≥ 15%) fracture risk status (FRS) by using the intervention threshold 15% as described in the JSBMR guideline.13) The ability of CTI in predicting the OPS and FRS was then analyzed to assess its usefulness in clinical practice.
All data analyses were performed by using IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA). The intraclass correlation coefficient (ICC) was calculated to assess intraand interobserver reliability of CFI and CTI measurements following the guideline by Koo and Li.14) The ICC was interpreted according to Landis and Koch as excellent (> 0.8), good (0.6–0.8), moderate (0.4–0.6) or poor (< 0.4).15) The Mann-Whitney U-test was used to evaluate the statistical differences in CFI, CTI and nBMD between males and females (reported as U, z, or p). The data were regrouped into male and female subgroups and the following analyses were performed in each subgroup: (1) Spearman correlation analysis (rho) was performed to estimate the correlation of nBMD and FRAX with CFI and CTI. Correlation strength was defined according to the study by Mukaka.16) (2) Based on the JSBMR guideline, subjects with previous femur or vertebral fractures would be diagnosed with osteoporosis regardless a BMD value.12,13) For that reason, we excluded those subjects from the OPS prediction analysis, leaving 424 subjects included. However, with the FRAX tool, these subjects were included since the tool could be used in patients receiving osteoporosis therapy, as reported by several publications.17) (3) Multiple regression analysis was performed to predict nBMD values from the CFI, CTI, height and weight parameters in subjects without previous femur or vertebral fractures. The effect size was also calculated. (4) Binary logistic regression analysis was performed to evaluate the ability of CTI to predict OPS in 424 subjects without previous femoral or vertebral fractures and to predict FRS in all 560 subjects. The new CTI10 variable, which was computed as 10 × CTI, was used to aid in better interpretation. Sensitivity (SEN), specificity (SPE), positive/negative predictive values and positive/negative likelihood ratios (+LR/−LR) were also calculated. The threshold values were computed from the regression equation with a cutoff probability of p = 0.5.
Table 1 shows the demographic data of the sample. Table 2 shows the median values of CTI, CFI, nBMD, and FRAX in the two sexes. Between males and females, there were significant differences with respect to CTI (U = 44.926, z = 3.861), CFI (U = 44.678, z = 3.724), nBMD (U = 59.505, z = 11.623) and FRAX (U = 11.619, z = −13.892) (Mann-Whitney U-test, all p < 0.001).
Intra- and interobserver reliability showed excellent results (ICCs ≥ 0.96, p < 0.001) for both CTI and CFI (Table 3). In both sexes, nBMD and FRAX showed statistically significant correlation with CTI and CFI (all p < 0.001). nBMD and FRAX were better correlated with CTI than CFI in both sexes (Table 4).
The regression analysis best-fit model comprised three variables including CTI, height, and weight (CFI was eliminated since p > 0.05). They statistically significantly predicted nBMD in females (F[3,331] = 96.67, size effect adjusted [adj] R2 = 46.2%) and males (F[3,221] = 42.84, adj R2 = 35.9%). The model for each sex had a significance of p < 0.001. The analysis showed that CTI had the greatest effect (β = 44.1% and β = 44.6% in females and males, respectively) on nBMD prediction among the three variables (Table 5).
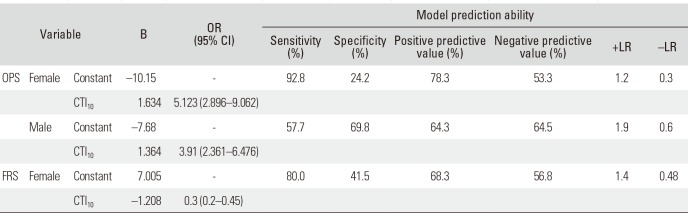
Binary logistic regression analysis was performed to ascertain the effects of CTI on the prediction of the likelihood of high-risk OPS and high-risk FRS. The OPS prediction model showed statistical significance for both females (χ2[1] = 39.77, p < 0.001) and males (χ2[1] = 19.62, p < 0.001), and the prediction ability is shown in Table 6. The CTI threshold values were calculated as 0.62 for females and 0.56 for males. The FRS prediction model showed significance for females only (χ2[1] = 41.01, p < 0.001): the CTI threshold value was calculated as 0.58 for females. By reducing the cutoff probability to p≈0.38, the value of 0.62 was calculated as a threshold value with better sensitivity.
In this cross-sectional study, CTI and CFI were correlated significantly with nBMD and FRAX in both sexes; nonetheless, CFI did not significantly contribute to nBMD and FRAX prediction. CTI, which had the better correlation with nBMD and FRAX, significantly predicted nBMD and FRAX values. In addition, the CTI also had the ability to predict OPS and FRS with good diagnostic ability, especially in females.
The excellent intra- and interobserver reliability of the method for measurement of CTI and CFI, regardless of observers' clinical experience, suggests that these two parameters can be reliably employed in clinical practice. The accuracy of CTI and CFI measurement has been proven in previous studies as well.81819) Besides, the duration of measurement is only within 1 minute. Thus, we propose that, in an outpatient setting, measuring CTI and CFI is much simpler and more practical than calculating FRAX. This would be especially useful in under-resourced countries where osteoporosis does not receive enough attention in orthopaedic practice because of the lack of DXA machine. Once having a hint from radiographs, orthopedists will pay more attention to osteoporosis-related issues and even consider spending more time to calculate FRAX and ordering DXA.
Our study observed significant differences in CTI, CFI, nBMD, and FRAX between males and females, implying the presence of sex-related dissimilarities in the data. Several epidemiologic and clinical studies have demonstrated significant differences between males and females regarding the prevalence of osteoporosis, fracture risk, mortality risk after fracture, and bone geometry and strength.2021222324) For this reason, we suggest that males and females should be studied separately on the issues related to osteoporosis.
Our data indicated that CTI and CFI were significantly correlated with nBMD and FRAX in both sexes (Table 4); between the two indices, CTI was correlated better with nBMD and FRAX than CFI, and this was consistent with other studies.2526) Yeung et al.11) demonstrated a weak correlation between CFI and T-score and a much stronger correlation between canal bone ratio (which is equal to 1 - CTI) and T-score. Other studies, by Sah et al.,26) Patterson et al.,18) and Baumgartner et al.,19) also confirmed a fairly high correlation between CTI and BMD.
Regression analysis was performed to evaluate the nBMD predicting ability of the different parameters. CFI was not found significantly contributing (p > 0.05) and eliminated from the regression model. Table 5 shows CTI as the parameter that affected nBMD the most. Our results correspond to those in the study by Webber et al.;27) however, the effect sizes of our model were larger (female, 46.2% and male, 35.9% vs. 15% for both sexes in that study).
In evaluating the capability of the predictive parameters to determine the dichotomous OPS (high-risk or normal) and FRS (high-risk or normal), the CTI threshold values of 0.62 for females and 0.56 for males were calculated with regard to OPS prediction (Table 6). While CTI in males (0.56 with SEN = 71.3% and SPE = 65.5%) shows moderate predictive ability and needs further examination, CTI in females (0.62 with SEN = 94.2% and −LR = 0.3) may be used as a preliminary screening tool for women who have the risk of having low BMD in clinical practice. Sah et al.26) suggested CTI ≤ 0.4 on a lateral hip radiograph as the threshold. However, their population sample included all postmenopausal osteoarthritic females undergoing hip replacement. The baseline BMD in this study population may have been elevated because of the reported inverse relationship between osteoporosis and osteoarthritis.282930) Patterson et al.18) proposed distal tibial cortical thickness value of 3.5 mm as the threshold value with 100% SEN and 100% negative predictive value. In their study, patient selection was cited as a limitation since the selected sample may have had a higher prevalence of osteoporosis than the general population.
On FRS prediction, CTI statistically significantly predicted FRS in females only, not in males. The CTI threshold value of 0.58 (females) was calculated from the model with SEN = 80% and SPE = 41.5 (Table 6). However, by raising the threshold value to 0.62 to collate with the threshold in the above OPS prediction model, the FRS prediction model demonstrated a better diagnostic ability with SEN = 95.6%, SPE = 20.8% and −LR = 0.2. Thus, the CTI value of 0.62 may also be considered as a preliminary screening tool for females whose FRAX values are high.
The major strength of our study was its large sample size. The study included a large number of volunteers aged ≥ 50 years to provide representative data of the general population within a geographic area, rather than of the subjects visiting the hospital.
Secondly, our study analyzed the two sexes separately. It helped not only reduce the errors associated with the demographic factor in analysis but also interpret the results with ease.
The primary limitation of our study was our selection criteria: people with a prior diagnosis of osteoporosis requiring medical therapy were included. This group represents a population with apparent osteoporosis, wherein CTI assessment is unnecessary. Although this may have altered the osteoporosis prevalence based on BMD, our data are more representative of a general population in reality. Moreover, our study was cross-sectional study; therefore, actual fracture incidence of the sample was not observed. Further longitudinal investigation may be conducted for more specific and meaningful data.
The second limitation is that all our volunteers were Japanese Asians and the data may be unrepresentative of other ethnicities. However, the complete data of DXA, FRAX and spine-hip X-ray make this sample valuable to investigate the hypothesis before proceeding onto other groups of people. Regarding the use of CTI in under-resourced countries, although the application of CTI to estimate fracture risk requires reference data, acquiring these data from a plenty of routinely ordered radiographs is more practical than performing DXA measurement in countries with lack of DXA. We will not discuss on how to obtain the reference data on a national scale since it may be more related to each nation's public health policy. However, on a hospital scale, we would like to propose a way of acquiring the data by conducting a longitudinal study as we are currently doing in our institution. The study consists of volunteers from the surrounding area of the hospital. Hip-spine radiographs will be taken every 2 years. Osteoporotic fractures will be recorded. Reference data will be concluded at the end of the study. We are now in our 5th year of the study.
The third limitation of our study is related to the position of the femur during radiography. The internal and external rotation of the femur while standing may affect the CTI and CFI measured on the anteroposterior radiographs. Lateral radiographs may be used for measurement but the limitation of femoral rotation still exists.
In conclusion, CTI of the proximal femur can be considered a reliable parameter that can be measured with ease on standard anteroposterior radiographs. It showed correlation and the ability to predict nBMD and FRAX at a statistically significantly level in the general elderly population. Our study proposes the use of CTI, in the case of lacking DXA machine for the assessment of BMD and FRAX, as a supportive assessment tool in estimating the risk of osteoporosis and fracture with radiography, especially in females.
References
1. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001; 285(6):785–795. PMID: 11176917.
2. Masi L. Epidemiology of osteoporosis. Clin Cases Miner Bone Metab. 2008; 5(1):11–13. PMID: 22460840.
3. Leboime A, Confavreux CB, Mehsen N, Paccou J, David C, Roux C. Osteoporosis and mortality. Joint Bone Spine. 2010; 77(Suppl 2):S107–S112. PMID: 21211746.

4. Hagino H, Sakamoto K, Harada A, et al. Nationwide one-decade survey of hip fractures in Japan. J Orthop Sci. 2010; 15(6):737–745. PMID: 21116890.

5. Kinoshita H, Tamaki T, Hashimoto T, Kasagi F. Factors influencing lumbar spine bone mineral density assessment by dual-energy X-ray absorptiometry: comparison with lumbar spinal radiogram. J Orthop Sci. 1998; 3(1):3–9. PMID: 9654549.

6. Mithal A, Ebeling P, Kyer CS. Asia-Pacific regional audit: epidemiology, costs and burden of osteoporosis in 2013 [Internet]. Nyon: International Osteoporosis Foundation;2013. cited 2018 Mar 9. Available from: https://www.iofbonehealth.org/datapublications/regional-audits/asia-pacific-regional-audit.
7. Noble PC, Box GG, Kamaric E, Fink MJ, Alexander JW, Tullos HS. The effect of aging on the shape of the proximal femur. Clin Orthop Relat Res. 1995; (316):31–44. PMID: 7634721.

8. Tawada K, Iguchi H, Tanaka N, et al. Is the canal flare index a reliable means of estimation of canal shape? Measurement of proximal femoral geometry by use of 3D models of the femur. J Orthop Sci. 2015; 20(3):498–506. PMID: 25740729.

9. Dorr LD, Faugere MC, Mackel AM, Gruen TA, Bognar B, Malluche HH. Structural and cellular assessment of bone quality of proximal femur. Bone. 1993; 14(3):231–242. PMID: 8363862.

10. Noble PC, Alexander JW, Lindahl LJ, Yew DT, Granberry WM, Tullos HS. The anatomic basis of femoral component design. Clin Orthop Relat Res. 1988; (235):148–165.

11. Yeung Y, Chiu KY, Yau WP, Tang WM, Cheung WY, Ng TP. Assessment of the proximal femoral morphology using plain radiograph-can it predict the bone quality? J Arthroplasty. 2006; 21(4):508–513. PMID: 16781402.

12. Soen S, Fukunaga M, Sugimoto T, et al. Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab. 2013; 31(3):247–257. PMID: 23553500.

13. Orimo H, Nakamura T, Hosoi T, et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis: executive summary. Arch Osteoporos. 2012; 7(1-2):3–20. PMID: 23203733.
14. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016; 15(2):155–163. PMID: 27330520.

15. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33(1):159–174. PMID: 843571.

16. Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012; 24(3):69–71. PMID: 23638278.
17. Leslie WD, Lix LM, Johansson H, et al. Does osteoporosis therapy invalidate FRAX for fracture prediction? J Bone Miner Res. 2012; 27(6):1243–1251. PMID: 22392538.

18. Patterson J, Rungprai C, Den Hartog T, et al. Cortical bone thickness of the distal part of the tibia predicts bone mineral density. J Bone Joint Surg Am. 2016; 98(9):751–760. PMID: 27147688.

19. Baumgartner R, Heeren N, Quast D, Babst R, Brunner A. Is the cortical thickness index a valid parameter to assess bone mineral density in geriatric patients with hip fractures? Arch Orthop Trauma Surg. 2015; 135(6):805–810. PMID: 25801811.

20. Diamantopoulos AP, Hoff M, Skoie IM, Hochberg M, Haugeberg G. Short- and long-term mortality in males and females with fragility hip fracture in Norway: a population-based study. Clin Interv Aging. 2013; 8:817–823. PMID: 23861581.

21. Thorne K, Johansen A, Akbari A, Williams JG, Roberts SE. The impact of social deprivation on mortality following hip fracture in England and Wales: a record linkage study. Osteoporos Int. 2016; 27(9):2727–2737. PMID: 27098537.

22. Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. 2011; 469(7):1900–1905. PMID: 21264553.

23. Kudlacek S, Schneider B, Resch H, Freudenthaler O, Willvonseder R. Gender differences in fracture risk and bone mineral density. Maturitas. 2000; 36(3):173–180. PMID: 11063899.

24. Kim TI, Choi JH, Kim SH, Oh JH. The adequacy of diagnosis and treatment for osteoporosis in patients with proximal humeral fractures. Clin Orthop Surg. 2016; 8(3):274–279. PMID: 27583110.

25. Mather J, MacDermid JC, Faber KJ, Athwal GS. Proximal humerus cortical bone thickness correlates with bone mineral density and can clinically rule out osteoporosis. J Shoulder Elbow Surg. 2013; 22(6):732–738. PMID: 23183030.
26. Sah AP, Thornhill TS, LeBoff MS, Glowacki J. Correlation of plain radiographic indices of the hip with quantitative bone mineral density. Osteoporos Int. 2007; 18(8):1119–1126. PMID: 17340218.

27. Webber T, Patel SP, Pensak M, Fajolu O, Rozental TD, Wolf JM. Correlation between distal radial cortical thickness and bone mineral density. J Hand Surg Am. 2015; 40(3):493–499. PMID: 25708436.

28. Hart DJ, Mootoosamy I, Doyle DV, Spector TD. The relationship between osteoarthritis and osteoporosis in the general population: the Chingford Study. Ann Rheum Dis. 1994; 53(3):158–162. PMID: 8154931.

29. Im GI, Kim MK. The relationship between osteoarthritis and osteoporosis. J Bone Miner Metab. 2014; 32(2):101–109. PMID: 24196872.

30. Dequeker J, Aerssens J, Luyten FP. Osteoarthritis and osteoporosis: clinical and research evidence of inverse relationship. Aging Clin Exp Res. 2003; 15(5):426–439. PMID: 14703009.

Fig. 1
Measurement of cortical thickness index (CTI) and canal flare index (CFI) on an anteroposterior radiograph (using Osirix software). Do: outer diameter (the shaft's outer diameter at 10 cm below the lesser trochanter), Di: inner diameter (the shaft's inner diameter at 10 cm below the lesser trochanter, measured at the same level as Do), CW: canal width (the canal width measured at 2 cm above Line a), Line a: a line drawn perpendicular to the femoral shaft through the middle point of the lesser trochanter, Line b: a line drawn parallel to the shaft to be used as a reference for drawing Line a, Line c: a 10-cm line drawn perpendicular to Line a, used to identify the shaft's inner and Do measurement levels.
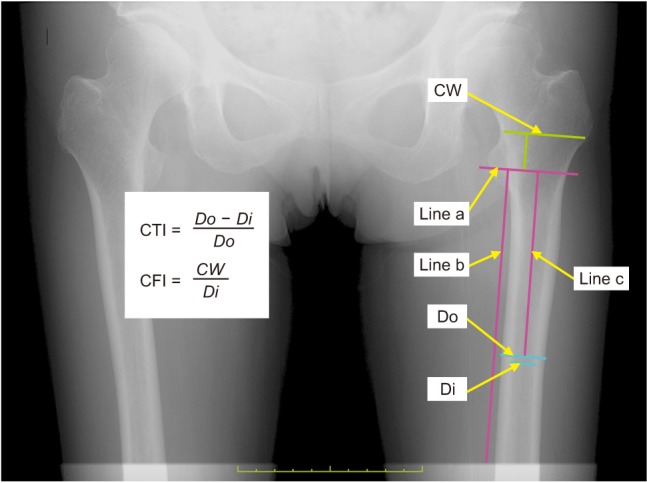
Table 1
Demographic Data
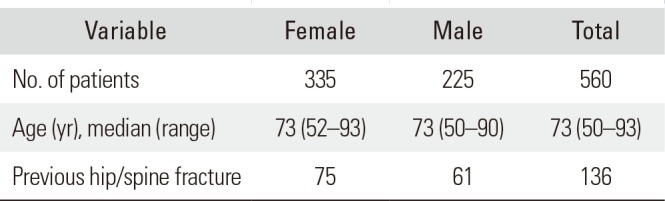
| Variable | Female | Male | Total |
|---|---|---|---|
| No. of patients | 335 | 225 | 560 |
| Age (yr), median (range) | 73 (52–93) | 73 (50–90) | 73 (50–93) |
| Previous hip/spine fracture | 75 | 61 | 136 |
Table 2
Median Values of CTI, CFI, nBMD and FRAX
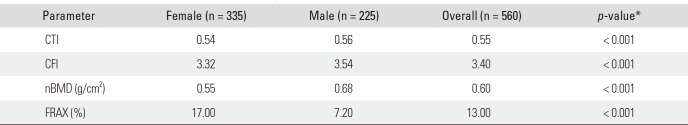
| Parameter | Female (n = 335) | Male (n = 225) | Overall (n = 560) | p-value* |
|---|---|---|---|---|
| CTI | 0.54 | 0.56 | 0.55 | < 0.001 |
| CFI | 3.32 | 3.54 | 3.40 | < 0.001 |
| nBMD (g/cm2) | 0.55 | 0.68 | 0.60 | < 0.001 |
| FRAX (%) | 17.00 | 7.20 | 13.00 | < 0.001 |
Table 3
ICC Values for Intra- and Interobserver Reliability
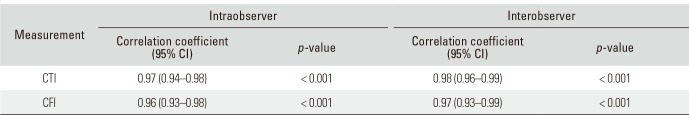
Table 4
Correlation between nBMD and CTI, CFI, HT, and WT
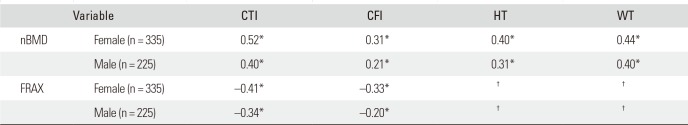
| Variable | CTI | CFI | HT | WT | |
|---|---|---|---|---|---|
| nBMD | Female (n = 335) | 0.52* | 0.31* | 0.40* | 0.44* |
| Male (n = 225) | 0.40* | 0.21* | 0.31* | 0.40* | |
| FRAX | Female (n = 335) | −0.41* | −0.33* | † | † |
| Male (n = 225) | −0.34* | −0.20* | † | † | |
Table 5
Regression* Coefficients of CTI, HT and WT in the nBMD Prediction Model
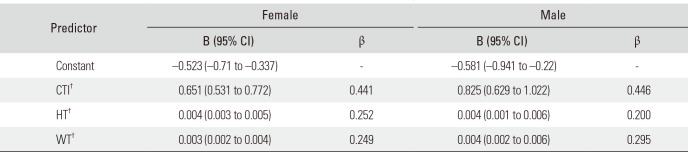
CTI: cortical thickness index, HT: height, WT: weight, nBMD: femoral neck bone mineral density, B: unstandardized coefficient, CI: confidence interval, β: standardized coefficient.
*Multiple linear regression analysis. †All three variables (CTI, HT, or WT) significantly added to the prediction (p < 0.001). The canal flare index was eliminated from the regression model (p > 0.05).
Table 6
Logistic Regression Calculation Using CTI to Predict OPS and FRS




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download