Abstract
Background
Chronic obstructive pulmonary disease is characterized by emphysema, chronic bronchitis, and small airway remodeling. The alveolar destruction associated with emphysema cannot be repaired by current clinical practices. Stem cell therapy has been successfully used in animal models of cigarette smoke- and elastase-induced emphysema. However, the optimal dose of mesenchymal stem cells (MSCs) for the most effective therapy has not yet been determined. It is vital to determine the optimal dose of MSCs for clinical application in emphysema cases.
Methods
In the present study, we evaluated the therapeutic effects of various doses of MSCs on elastase-induced emphysema in mice. When 3 different doses of MSCs were intravenously injected into mice treated with elastase, only 5×104 MSCs showed a significant effect on the emphysematous mouse lung. We also identified action mechanisms of MSCs based on apoptosis, lung regeneration, and protease/antiprotease imbalance.
Results
The MSCs were not related with caspase-3/7 dependent apoptosis. But activity of matrix metalloproteinase 9 increased by emphysematous lung was decreased by intravenously injected MSCs. Vascular endothelial growth factor were also increased in lung from MSC injected mice, as compared to un-injected mice.
Chronic obstructive pulmonary disease (COPD) characterized by emphysema, chronic bronchitis, and small airway remodeling is one of the leading causes of death worldwide12. Cigarette smoking is a chief causative agent in the development of COPD characterized by progressive alveolar destruction and persistent inflammation3. Emphysema is progressively marked by alveolar destruction and degradation of the extracellular matrix by protease-antiprotease imbalance and abnormal apoptosis and repair of resident lung cells45. Although some drugs exist that reduce airway obstruction in patients with COPD, it is difficult to regenerate damaged alveolar structures or lung tissue67.
Our recent research has shown that bone marrow-derived mesenchymal stem cells (MSCs) are able to repair damage from cigarette smoke-induced emphysema8. Other research has also observed that MSCs from bone marrow and adipose tissue have a therapeutic effect in cigarette smoke- and elastase-induced emphysema910. Although most research studies to evaluate the therapeutic effect of MSCs in treating emphysema have applied intravenous injection, the optimal therapeutic dose of MSCs in a mouse model of emphysema remains unclear.
MSCs have been isolated from bone marrow, adipose tissue, umbilical cord blood, and placenta1112. MSCs are considered to have therapeutic capacities in a spectrum of diseases through paracrine, anti-inflammatory, immunomodulatory, and anti-apoptotic properties13. Recent reports have shown that different doses of MSCs exert different effects on healing or cytokine expression in a non-dose dependent manner14. However, another study reported that MSCs injected in the early period following acute myocardial infarction showed a significant effect and demonstrated dose dependence15.
Although it is important to determine the optimal doses of MSCs in treating emphysema, no such research has been done. Using an animal model is especially useful in determining the optimal dose of MSCs for clinical application in emphysema cases. In the present study, we intravenously injected different doses of cord blood MSCs into mice with elastase-induced emphysema and evaluated the therapeutic effects following each dose and the detailed action mechanisms of MSCs.
C57BL/6J mice were purchased from Orientbio (Seongnam, Korea). Mice were bred in specific pathogen-free facilities at Asan Medical Center. All live mouse experiments were approved by the Institutional Animal Care and Use Committee of Asan Medical Center (Korea). Female C57BL/6J mice (18-20 g, 7 weeks of age) were intratracheally injected with 0.4 U of porcine pancreatic elastase (Sigma-Aldrich, St. Louis, MO, USA) at day 0, were intravenously injected with different doses of MSCs at day 7, and were sacrificed at day 14.
Human umbilical cord blood-derived MSCs were provided by MEDIPOST (Seoul, Korea). The phenotype and differentiation ability of the MSCs were already confirmed by MEDIPOST. The MSCs were maintained with alpha-minimum essential medium (Gibco, Carlsbad, CA, USA), were replenished with fresh media twice a week, and were subcultured using 0.25% trypsin-EDTA (Gibco).
The lungs were perfused with phosphate buffered saline and inflated by intratracheal infusion of 0.5% low-melting agar at 25 cm H2O, fixed in 4% paraformaldehyde, and embedded in paraffin. Lung sections of 4 µm thickness were stained with hematoxylin and eosin. The mean linear intercepts (MLI) were determined separately by two investigators in a blinded fashion8.
Proteins from lung tissue were prepared with a cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA) and quantified based on the Bradford assay. The 10 µg of protein were incubated with caspase-3/7 substrate diluted with caspase-3/7 buffer in black multiwell plates (Promega, Madison, WI, USA). After 5 hours, the fluorescence of each sample was measured using a fluorometer.
The mRNA from the lung tissue was prepared using the RNeasy Mini Kit (Qiagen, Duesseldorf, Germany) and quantified using spectrophotometer (NanoDrop Technologies, Rockland, DE, USA), and the cDNA was then synthesized using a cDNA synthesis kit (Thermo Scientific, Waltham, MA, USA). Quantitative polymerase chain reaction (PCR) analyses for matrix metalloproteinase (MMP) 2, MMP9, MMP12, SLIP, and tissue inhibitor of metalloproteinases-1 (TIMP1) were performed using a real-time LightCycler 480 PCR system (Roche Diagnostics, Basel, Switzerland) with LightCycler 480 SYBR Green I master (Roche Diagnostics). The primers used for MMP2, MMP9, MMP12, SLIP, and TIMP1 were displayed in Table 1.
We measured MMP2 and MMP9 activity using gelatin zymography as previously described16. The lung homogenate were prepared with cell lysis buffer without protease inhibitor. The 10 µg of protein were loaded into sodium dodecyl sulfate polyacrylamide gel electrophoresis gel containing gelatin at a concentration 2.6 mg/mL. Gel were then incubated in 2.5% Triton X-100 and incubated for overnight in zymogaphy developing buffer at 37℃. And then gel were stained and destained with 0.5% comassie G250 in 30% ethanol/10% acetic acid and 30% ethanol/10% acetic acid, respectively.
The 10 µg of protein from lung tissue were examined by enzyme-linked immunosorbent assay (ELISA). The hepatocyte growth factor (HGF), fibroblast growth factor 2 (FGF2), and vascular endothelial growth factor (VEGF) levels in the lung lysates were measured by ELISA according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA).
To identify the optimal dose of MSCs for treatment of emphysema, C57BL/6J mice were treated intratracheally with 0.4 U of elastase on day 0 and were intravenously injected with 1×104, 2.5×104, 5×104, or 1×105 of MSCs on day 7. Lung tissue was then collected on day 14. The mice treated with elastase only showed severe alveolar destruction compared to the control group, while the mice treated with MSCs showed less alveolar destruction (Figure 1A). The level of alveolar destruction was quantified by measuring MLI. The mice treated with the 5×104 MSCs showed significantly lower MLI (78.15 µm) than the mice treated with elastase only (92.28 µm) (Figure 1B). The mice treated with the 1×104 MSCs, 2.5×104, or 1×105 of MSCs also showed a pattern of decreased MLI (81.82 µm, 88.75 µm, or 83.47 µm, respectively) compared to the mice treated with elastase only, but the difference was not significant (Figure 1B).
To identify action mechanisms of MSCs in this model of elastase-induced emphysema, we obtained protein and mRNA in lung tissue from three of the mouse groups (control, elastase-only, and elastase- and 5×104 MSC-treated groups). The therapeutic mechanisms are divided in apoptosis, protease/antiprotease and regeneration by growth factor. First, we test caspase 3/7 dependent apoptosis. Caspase 3/7 activity to detect cellular apoptosis is not significantly different among the three groups (Figure 2). Second, we performed an evaluation based on protease/antiprotease imbalance in the same three groups. The mRNA expression of MMP2, MMP9, and MMP12 were measured using quantitative PCR, and data were presented as a ratio of the control group (Figure 3A). In the mouse group treated with elastase only, mRNA expression of MMP12 was higher than in the control group. The mouse group treated with both elastase and MSCs may be also higher than in the control group but there is not statistical difference. Next, we performed gelatin zymography to observe the activity of MMP2 and MMP9 (Figure 3B). The activity of MMP2 and MMP9 were induced in elastase only treated mouse group compared to the control group. In elastase and MSC treated mouse group, the MMP9 activity was decreased compared with the elastase only treated mouse group. The mRNA expression of SLPI and TIMP1 (representative antiprotease) were measured by quantitative PCR, and data were displayed as a ratio of the control group (Figure 3C). SLPI and TIMP1 expression in lung is not significantly different among the three groups. HGF, FGF2, and VEGF production related to the lung regeneration was also measured in lung homogenate using ELISA. HGF and FGF2 were not significantly different among the three groups (Figure 4A, B). The VEGF in lung tissue were decreased in elastase only treated mouse group compared to control mouse group and were recovered in elastase and MSCs treated mouse group (Figure 4C).
In this study, we identified the optimal dose of MSCs for therapy in a mouse model of elastase-induced emphysema. A dose of 5×104 MSCs showed significant therapeutic effects in elastase-induced emphysema in C57BL/6J mice. A lower dose of 1×104 or 2.5×104 MSCs also showed slightly therapeutic effects but not to a significant degree. Moreover, we evaluated the action mechanisms of the dosage of 5×104 MSCs in elastase-induced emphysema. The effects of MSCs in emphysematouse lung were reduction of MMP9 activity and induction of VEGF production. Therefore, there is best therapeutic dose of MSCs in emphysema and these result may be become basic data to decide clinical application for patients with emphysema.
Some reports have shown that it is possible to apply stem cells as an effective therapy to regenerate destroyed alveolar structures8910. Our previous report has shown that bone marrow cells (6×106) and MSCs (6×105) have the capability of repairing cigarette smoke-induced emphysema in rats exposed to cigarette smoke for 6 months8. Other researchers have observed positive effects in mice from the injection of adipose-derived stem cells (3×105) every other week during 3 or 4 months of cigarette smoke exposure10. In the mouse model of elastase-induced emphysema, 2.8×106 bone marrow mononuclear cells can repair destroyed alveolar structures9. There is no standard therapeutic dose of stem cells in the animal model of emphysema/COPD. These results represent the first report on the optimal dose of MSCs for the treatment of emphysema.
A recent report showed that when different doses of MSCs were injected into the injured ligaments of two groups of rats, the higher dose group exhibited increased expression of pro-inflammatory cytokines, including interleukin (IL)-1β, interferon γ, IL-2, while the lower dose group showed enhanced functional healing compared to the higher dose group14. It is noteworthy that in most clinical trials of MSCs, the amount of MSCs injected depends on the patient's body weight171819. Thus, patients with higher body weights will require more MSCs and incur greater costs. Determining the optimal dose of MSCs is vital because of a variety of factors, such as immune response, inflammatory cytokines, therapeutic effects, and financial burden.
Animal models for COPD have been developed through the agents of cigarette smoke and elastase. An elastase-induced emphysema model is widely applied, and supports the hypothesized role of protease/antiprotease imbalance in the development of emphysema. Although a mouse model of elastase-induced emphysema is not perfectly representative of human COPD/emphysema, this model focuses on alveolar destruction based on protease/antiprotease imbalance. Therefore, we observed cellular apoptosis as measured by caspase 3/7 enzyme activity, and found that it was not higher in the elastase only and elastase and MSCs treated mouse group compared to the control mouse group. The induction of MMP12 mRNA expression was observed in the elastase only treated mouse group. MMPs is more important their activity than mRNA expression. Especially, it was well known about MMP2 and MMP9 activity linked with asthmatic and COPD patients16. These results are shown in decrease of MMP9 activity because of MSCs therapeutic effects.
Many other reports suggest that the paracrine properties of MSCs enable a key mechanism of action in the treatment of emphysema. In fact, it is well known that MSCs produce many cytokines and growth factors1320. These include some candidate molecules in the categories of growth factors (HGF, keratinocyte growth factor, FGF2, and VEGF) and cytokines (IL-10). Although inflammation is a very important factor in the development of emphysema/COPD, we applied MSCs following complete recovery from inflammation. Therefore, this study did not confirm the anti-inflammatory effect of IL-10. HGF is a viable candidate growth factor for the repair of lung destruction, as intranasally administered HGF has been shown to ameliorate the effects of elastase-induced emphysema in mice21. The other report demonstrated that growth factors derived from human MSCs were decreased in SLPI expression in mouse lung epithelial cells22. FGF2 is an important component of the regulation of lung development and need to proliferation and migration of alveolar epithelial cell23. Therefore, FGF2 is also critical to repair of lung injury. In this study, although MSCs showed a therapeutic effect on elastase-induced emphysema, MSCs were not found to increase HGF and FGF2 protein levels.
VEGF is important role during airway growth especially, development and maintenance of the pulmonary vasculature23. Moreover, inhibition of VEGF receptor in animal leads to emphysema features including alveolar space enlargement and alveolar cell apoptosis24. Recent report was shown that MSCs protect cigarette smoked lung damage partly via VEGF-VEGF receptors25. In this report, they observed that VEGF from MSCs was crucial for protection of lung damage. MSCs can induce the expression of VEGF and their receptors and enhanced VEGF signal prevent lung damage by cigarette smoking.
In clinical trial, MSCs were infused four monthly 100×106 cells into patient with COPD or control26. Systemic injection of MSCs was not significant improved in pulmonary function test or quality of life. Clinical trial for other diseases, some researchers applied with MSCs depending on patient's weight such as (2-10)×106 cells/kg and (5.7-7.5)×108 cells/kg for Hurler syndrome and Osteogenesis imperfecta, respectively2728. Although additional researches need to determine MSCs dose for clinical trial, we suggest that optimal dose of MSCs in preclinical emphysema model be able to apply to clinical trial ratio to weight.
In conclusion, we report here for the first time that there is an optimal dose of MSCs for the treatment of elastase-induced emphysema in mice. And the therapeutic effects of MSCs are related with decrease of MMP9 activity and increase of VEGF production. Our report will provide important basic data for deciding on dosage in clinical application of MSCs.
Figures and Tables
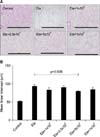 | Figure 1Optimal dose of mesenchymal stem cells (MSCs) to repair elastase induced emphysema in mice. C57BL/6J mice were administered 0.4 U of elastase on day 0 by intratracheal application and then intravenously injected with MSCs on day 7. Data are measures of the histological staining with hematoxylin and eosin in lung section on day 14. (A) Control (n=5), Ela (elastase only, n=15), Ela+1×104 (elastase+1×104 MSC, n=10), Ela+2.5×104 (elastase+2.5×104 MSC, n=10), Ela+5×104 (elastase+5×104 MSC, n=16), and Ela+1×105 (elastase+1×105 MSC, n=6) (×10). Scale bars=1.0 mm. (B) Morphometic analysis of the mean linear intercept. Values are presented as the mean±SEM. |
 | Figure 2The anti-apoptotic effects of mesenchymal stem cell (MSC) in elastase induced emphysema. C57BL/6J mice were intratracheally applied with 0.4 U of elastase on day 0 and then intravenously injected with MSCs on day 7. Lung tissue were collected on day 14 (n=6-9 per group). Caspase 3/7 activity was measured with fluorimetric emzymatic assay and normalized by protein concentration in lung homogenates. Ela: elastase only; Ela+5×104: elastase+5×104 MSC. |
 | Figure 3The effects on protease and anti-protease in elastase induced emphysema with or without mesenchymal stem cells (MSCs). C57BL/6J mice were intratracheally applied with 0.4 U of elastase on day 0 and then intravenously injected with MSCs on day 7. Lung tissues were collected on day 14 (n=6-9 per group). (A) Matrix metalloproteinase (MMP) 2, MMP9, and MMP12 mRNA expression were measured with quantitative polymerase chain reaction (PCR) using SYBR Green and normalized by β-actin expression and then displayed with ratio to control group. Values are presented as the mean±SEM. *p<0.05, as compared with control group. (B) Activity of MMP2 and 9 were measured with gelatin zymography. (C) The mRNA expression of SLPI (left) and tissue inhibitor of metalloproteinases-1 (TIMP1) (right) were measured with quantitative PCR using SYBR Green and normalized by β-actin expression and then displayed with ratio to control group. PBS: phosphate buffered saline; Ela: elastase only; Ela+5×104: elastase+5×104 MSC. |
 | Figure 4The production of growth factors in elastase induced emphysema with or without mesenchymal stem cells (MSCs). C57BL/6J mice were intratracheally applied with 0.4 U of elastase on day 0 and then intravenously injected with MSCs on day 7. Lung tissue were collected on day14 (n=5-9 per group). The growth factors were measured with enzyme-linked immunosorbent assay for hepatocyte growth factor (HGF) (A), fibroblast growth factor 2 (FGF2) (B), and vascular endothelial growth factor (VEGF) (C). Values are presented as the mean±SEM. *p<0.05 was statistical significance of the comparison between 2 groups. Ela: elastase only; Ela+5×104: elastase+5×104 MSC. |
Table 1
Primer list

Acknowledgements
The authors thank the members of the Asan Medical Center animal facility for their technical expertise. This study was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (no. HI12C0169).
References
1. Senior RM, Anthonisen NR. Chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. 1998; 157(4 Pt 2):S139–S147.
2. Sutherland ER, Cherniack RM. Management of chronic obstructive pulmonary disease. N Engl J Med. 2004; 350:2689–2697.
3. Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol. 2008; 294:L612–L631.
4. Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest. 2000; 117:684–694.
5. Yokohori N, Aoshiba K, Nagai A. Respiratory Failure Research Group in Japan. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest. 2004; 125:626–632.
6. Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007; 146:545–555.
7. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356:775–789.
8. Huh JW, Kim SY, Lee JH, Lee JS, Van Ta Q, Kim M, et al. Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol. 2011; 301:L255–L266.
9. Longhini-Dos-Santos N, Barbosa-de-Oliveira VA, Kozma RH, Faria CA, Stessuk T, Frei F, et al. Cell therapy with bone marrow mononuclear cells in elastase-induced pulmonary emphysema. Stem Cell Rev. 2013; 9:210–218.
10. Schweitzer KS, Johnstone BH, Garrison J, Rush NI, Cooper S, Traktuev DO, et al. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med. 2011; 183:215–225.
11. Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006; 24:1294–1301.
12. Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal 'stem' cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003; 121:368–374.
13. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013; 45:e54.
14. Saether EE, Chamberlain CS, Leiferman EM, Kondratko-Mittnacht JR, Li WJ, Brickson SL, et al. Enhanced medial collateral ligament healing using mesenchymal stem cells: dosage effects on cellular response and cytokine profile. Stem Cell Rev. 2014; 10:86–96.
15. Richardson JD, Bertaso AG, Psaltis PJ, Frost L, Carbone A, Paton S, et al. Impact of timing and dose of mesenchymal stromal cell therapy in a preclinical model of acute myocardial infarction. J Card Fail. 2013; 19:342–353.
16. Cataldo D, Munaut C, Noel A, Frankenne F, Bartsch P, Foidart JM, et al. MMP-2- and MMP-9-linked gelatinolytic activity in the sputum from patients with asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 2000; 123:259–267.
17. Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh WI, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr. 2014; 164:966–972.e6.
18. Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009; 54:2277–2286.
19. Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010; 69:1423–1429.
20. Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996; 166:585–592.
21. Hegab AE, Kubo H, Yamaya M, Asada M, He M, Fujino N, et al. Intranasal HGF administration ameliorates the physiologic and morphologic changes in lung emphysema. Mol Ther. 2008; 16:1417–1426.
22. Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T, et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther. 2011; 19:196–203.
23. Muyal JP, Muyal V, Kotnala S, Kumar D, Bhardwaj H. Therapeutic potential of growth factors in pulmonary emphysematous condition. Lung. 2013; 191:147–163.
24. Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000; 106:1311–1319.
25. Guan XJ, Song L, Han FF, Cui ZL, Chen X, Guo XJ, et al. Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary function partly via VEGF-VEGF receptors. J Cell Biochem. 2013; 114:323–335.
26. Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013; 143:1590–1598.
27. Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant. 2002; 30:215–222.
28. Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999; 5:309–313.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download