Abstract
Objective
To evaluate the association between circulating adiponectin, visfatin, and ghrelin levels and systemic lupus erythematosus (SLE).
Methods
We conducted a meta-analysis to compare serum/plasma adiponectin, visfatin, and ghrelin levels in patients with SLE to those of healthy controls.
Results
Eleven articles (822 patients with SLE and 676 controls) were included in the meta-analysis. The meta-analysis showed that the adiponectin level was significantly higher in the SLE group than in the control group (standardized mean difference [SMD]=0.360, 95% confidence interval [CI]=0.025∼0.695, p=0.035). Stratification according to region showed that high adiponectin levels were associated with SLE in the Western population (SMD=0.225, 95% CI=0.024∼0.426, p=0.028), but not in the South American population. A subgroup analysis that adiponectin level is significantly higher in the SLE group than in the control after adjustment for age, sex, body mass index, large sample size (n>100); and mean age>40 years (SMD=0.492, 95% CI=0.065∼0.920, p=0.024; SMD=0.492, 95% CI=0.065∼0.920, p=0.024; SMD=0.429, 95% CI=0.124∼0.733, p=0.006, respectively). Stratification by region showed significantly increased visfatin and ghrelin levels in the SLE group in Western and South American populations.
Go to : 
REFERENCES
1. Ruiz-Irastorza G, Khamashta MA, Castellino G, Hughes GR. Systemic lupus erythematosus. Lancet. 2001; 357:1027–32.

2. Shao WH, Cohen PL. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res Ther. 2011; 13:202.

3. Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013; 9:191–200.
4. Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens). 2012; 11:8–20.

5. Cheng X, Folco EJ, Shimizu K, Libby P. Adiponectin induces proinflammatory programs in human macrophages and CD4+ T cells. J Biol Chem. 2012; 287:36896–904.

6. Lee YA, Ji HI, Lee SH, Hong SJ, Yang HI, Chul Yoo M, et al. The role of adiponectin in the production of IL-6, IL-8, VEGF and MMPs in human endothelial cells and osteoblasts: implications for arthritic joints. Exp Mol Med. 2014; 46:e72.

7. Tang CH, Chiu YC, Tan TW, Yang RS, Fu WM. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J Immunol. 2007; 179:5483–92.
8. Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008; 83:804–16.

9. Brentano F, Schorr O, Ospelt C, Stanczyk J, Gay RE, Gay S, et al. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis Rheum. 2007; 56:2829–39.

10. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000; 141:4255–61.
11. Karmiris K, Koutroubakis IE, Kouroumalis EA. Leptin, adiponectin, resistin, and ghrelin–implications for inflammatory bowel disease. Mol Nutr Food Res. 2008; 52:855–66.
12. Barbosa Vde S, Francescantônio PL, Silva NA. Leptin and adiponectin in patients with systemic lupus erythematosus: clinical and laboratory correlations. Rev Bras Reumatol. 2015; 55:140–5.
13. McMahon M, Skaggs BJ, Grossman JM, Sahakian L, Fitzgerald J, Wong WK, et al. A panel of biomarkers is associated with increased risk of the presence and progression of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheumatol. 2014; 66:130–9.

14. Vadacca M, Zardi EM, Margiotta D, Rigon A, Cacciapaglia F, Arcarese L, et al. Leptin, adiponectin and vascular stiffness parameters in women with systemic lupus erythematosus. Intern Emerg Med. 2013; 8:705–12.

15. Reynolds HR, Buyon J, Kim M, Rivera TL, Izmirly P, Tunick P, et al. Association of plasma soluble E-selectin and adiponectin with carotid plaque in patients with systemic lupus erythematosus. Atherosclerosis. 2010; 210:569–74.

16. De Sanctis JB, Zabaleta M, Bianco NE, Garmendia JV, Rivas L. Serum adipokine levels in patients with systemic lupus erythematosus. Autoimmunity. 2009; 42:272–4.

17. Chung CP, Long AG, Solus JF, Rho YH, Oeser A, Raggi P, et al. Adipocytokines in systemic lupus erythematosus: relationship to inflammation, insulin resistance and coronary atherosclerosis. Lupus. 2009; 18:799–806.

18. Al M, Ng L, Tyrrell P, Bargman J, Bradley T, Silverman E. Adipokines as novel biomarkers in paediatric systemic lupus erythematosus. Rheumatology (Oxford). 2009; 48:497–501.

19. Sada KE, Yamasaki Y, Maruyama M, Sugiyama H, Yamamura M, Maeshima Y, et al. Altered levels of adipocytokines in association with insulin resistance in patients with systemic lupus erythematosus. J Rheumatol. 2006; 33:1545–52.
20. Rovin BH, Song H, Hebert LA, Nadasdy T, Nadasdy G, Birmingham DJ, et al. Plasma, urine, and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 2005; 68:1825–33.

21. Ozgen M, Koca SS, Aksoy K, Dagli N, Ustundag B, Isik A. Visfatin levels and intima-media thicknesses in rheumatic diseases. Clin Rheumatol. 2011; 30:757–63.

22. Kim HA, Choi GS, Jeon JY, Yoon JM, Sung JM, Suh CH. Leptin and ghrelin in Korean systemic lupus erythematosus. Lupus. 2010; 19:170–4.

23. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Associations between osteoprotegerin polymorphisms and bone mineral density: a meta-analysis. Mol Biol Rep. 2010; 37:227–34.

24. Lee YH, Rho YH, Choi SJ, Ji JD, Song GG. PADI4 polymorphisms and rheumatoid arthritis susceptibility: a meta-analysis. Rheumatol Int. 2007; 27:827–33.

25. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Induction and maintenance therapy for lupus nephritis: a systematic review and meta-analysis. Lupus. 2010; 19:703–10.

26. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997; 40:1725.

27. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.

28. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005; 5:13.

29. Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR. Depression and telomere length: A meta-analysis. J Affect Disord. 2016; 191:237–47.

30. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa (ON): Ottawa Hospital Research Institute;2000. [cited 2016]. Available from:. http://www.ohri.-ca/programs/clinical_epidemiology/oxford.asp.
31. McGough JJ, Faraone SV. Estimating the size of treatment effects: moving beyond p values. Psychiatry (Edgmont). 2009; 6:21–9.
32. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997; 315:1533–7.

34. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–58.

35. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34.

36. Li HM, Zhang TP, Leng RX, Li XP, Li XM, Pan HF. Plasma/serum leptin levels in patients with systemic lupus erythematosus: a meta-analysis. Arch Med Res. 2015; 46:551–6.

37. Sun Z, Lei H, Zhang Z. Pre-B cell colony enhancing factor (PBEF), a cytokine with multiple physiological functions. Cytokine Growth Factor Rev. 2013; 24:433–42.

38. Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007; 178:1748–58.

Go to : 
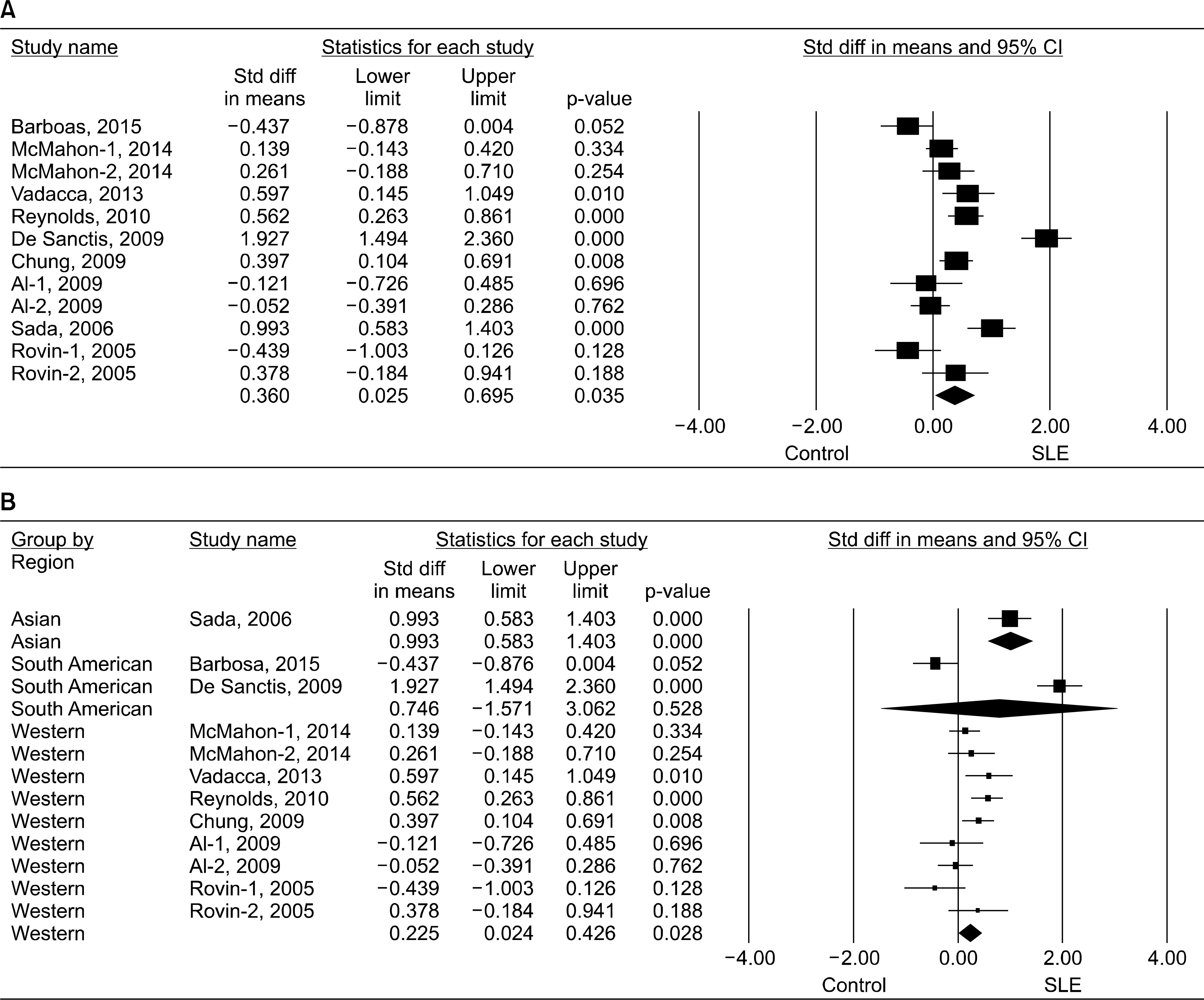 | Figure 1.Meta-analysis of the relationship between adiponectin and systemic lupus erythematosus (SLE) in all study subjects (A) and each study region (B). Std diff: standardized difference, CI: confidence interval. |
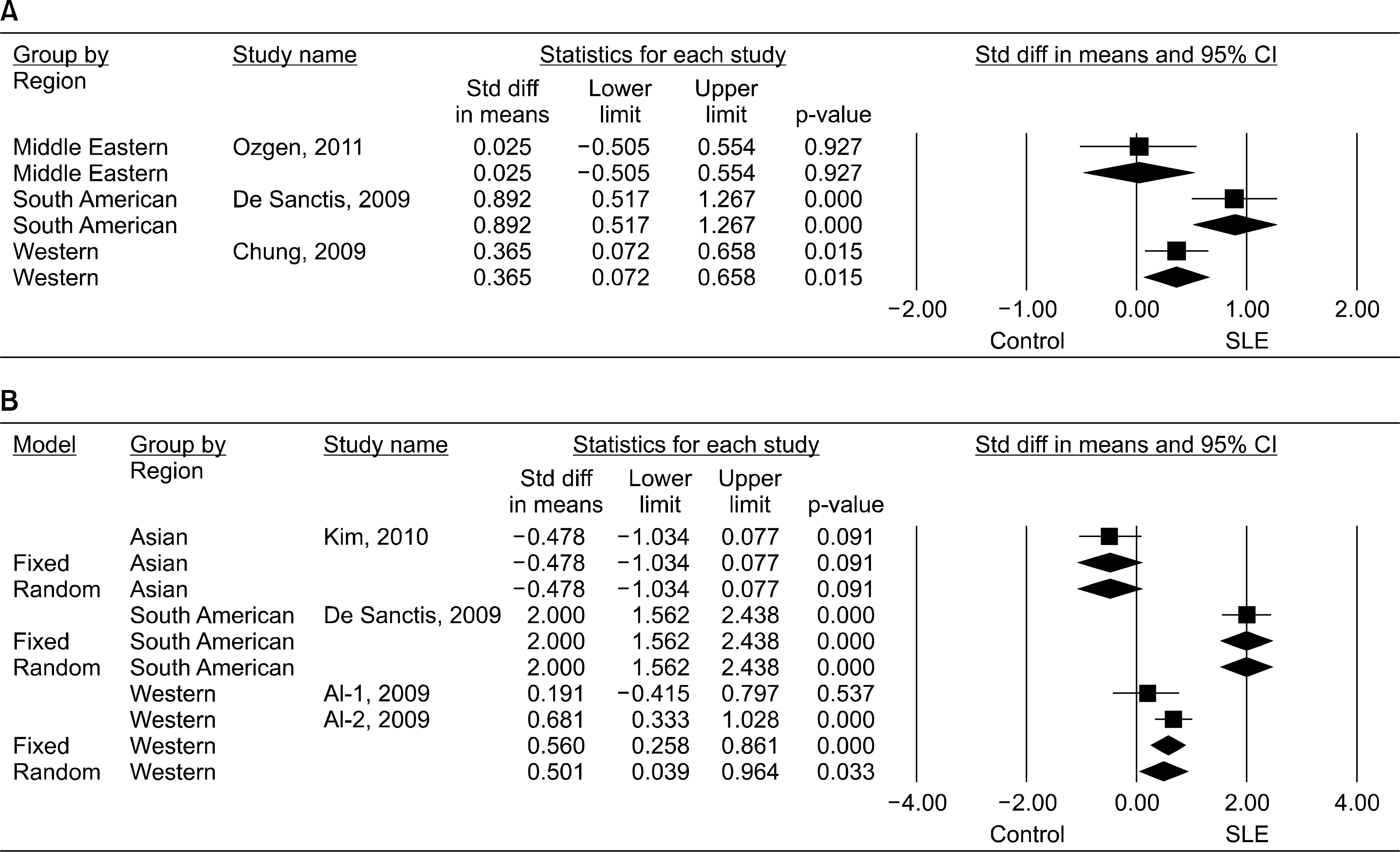 | Figure 2.Meta-analysis of the relationship between visfatin (A) or ghrelin (B) levels and systemic lupus erythematosus (SLE) in each study region. Std diff: standardized difference, CI: confidence interval. |
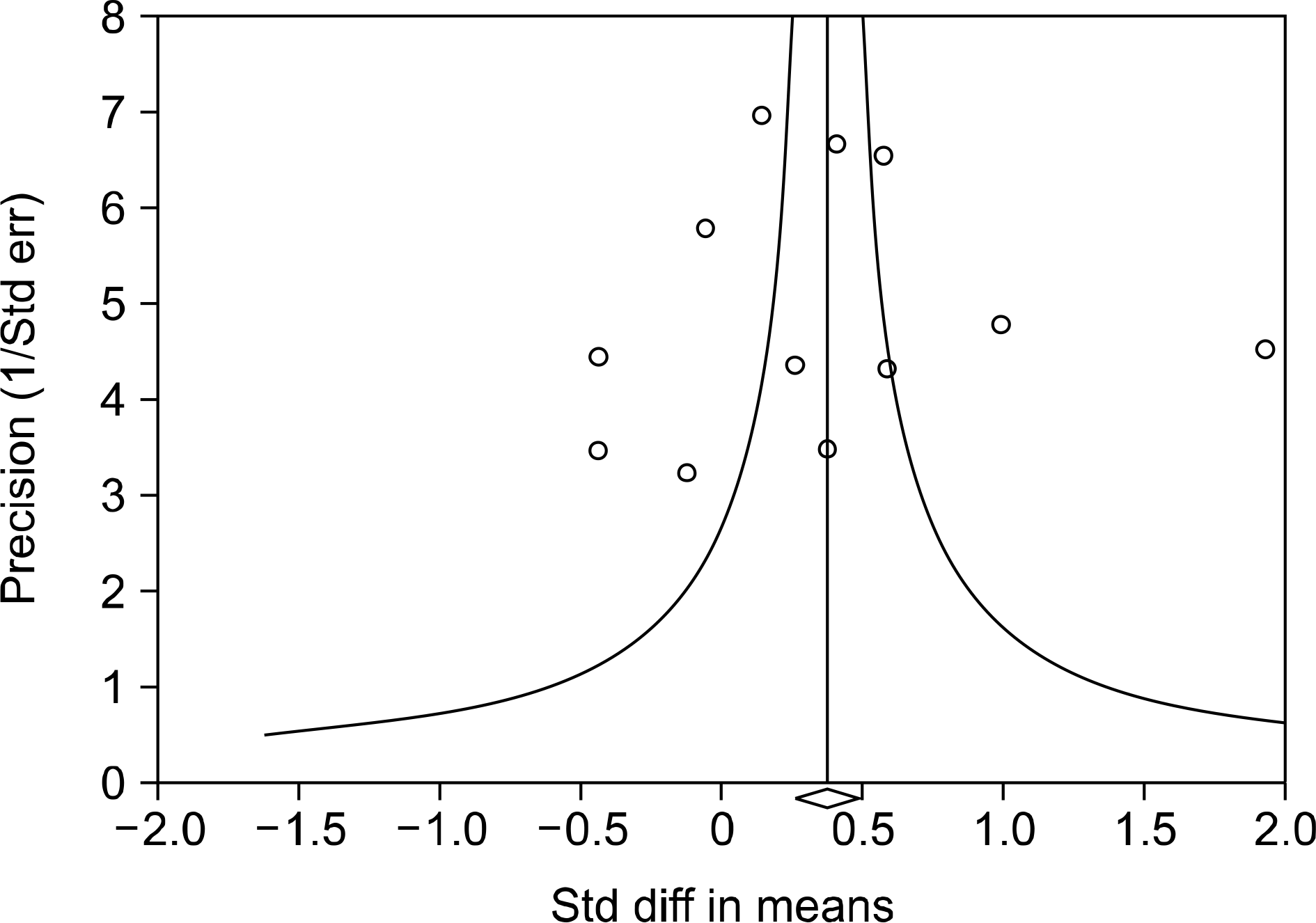 | Figure 3.Funnel plot of studies that examined the relationship between adiponectin and systemic lupus erythematosus (Egger regression p-value=0.894). Std diff: standardized difference, Std err: standardized error. |
Table 1.
Characteristics of individual studies included in the meta-analysis
| Study | Country | Patient | Age, yr | BMI, kg/m2 | Disease duration, yr | Adipokines | Adjustment* | Quality score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SLE | Control | SLE | Control | SLE | Control | ||||||
| Barbosa, 2015 [12] | Brazil | 52 | 33 | 33.4±9.4 | 32.5± 10.5 | 23.8+3.5 | 21.8+2.5 | 7.5 | A | Age | 8 |
| McMahon-1, 2014 [13] | USA | 142 | 72 | 39.6±13.5 | 40.5±11.8 | 25.7+5.9 | 23.7+5.1 | 11.4 + 8.0 | A | Age | 7 |
| McMahon-2, 2014 [13] | USA | 61 | 28 | 51.9±10.2 | 54.6± 10.1 | 28.0+7.1 | 25.3+5.8 | 14.9+11.4 | A | Age | 7 |
| Vadacca, 2013 [14] | Italy | 60 | 29 | 42.26±40.54 | 45.69± 11.57 | 25.2+4 | 24+4.6 | 10+5 | A | Age, gender, BMI | 9 |
| Reynolds, 2010 [15] | USA | 119 | 71 | 42.6±41.3 | 41.3 + 11.9 | 25.9+6.3 | 24.8+5.3 | NA | A | Age, gender, BMI | 8 |
| De Sanctis, 2009 [16] | Venezuela | 60 | 60 | 36±6 | 32±12 | 24+2.7 | 22+2.0 | NA | A, V, G | NA | 6 |
| Chung, 2009 [17] | USA | 109 | 78 | 40.2±11.5 | 40.5 + 12.0 | 29.2+7.5 | 27.0+6.0 | 8.2+7.3 | A, V | Age, gender | 9 |
| Al-1, 2009 [18] | Canada | 21 | 21 | 14.43±2.20 | 10.04 + 3.48 | 24.07+3.57 | NA | NA | A, G | Gender | 7 |
| Al-2, 2009 [18] | Canada | 84 | 56 | 14.32±2.67 | 10.04 + 3.48 | 23.76+5.30 | NA | NA | A, G | Gender | 7 |
| Sada, 2006 [19] | Japan | 37 | 80 | 44±15 | 44±6 | 22.1+3.5 | 22.2+3.2 | 9.4+7.1 | A | Age, gender, BMI | 8 |
| Rovin-1, 2005 [20] | USA | 18 | 39 | 47.9±7.21 | 33.5 + 10.6 | 32.6 + 6.36 | 25.9 + 5.09 | NA | A | NA | 6 |
| Rovin-2, 2005 [20] | USA | 18 | 39 | 34.6±7.64 | 33.5 + 10.6 | 29.5 + 10.62 | 25.9 + 5.09 | NA | A | NA | 6 |
| Ozgen, 2011 [21] | Turkey | 26 | 29 | 34.2±11.0 | 38.0+10.3 | 23.2+4.4 | 25.9+4.7 | 3.8 + 3.9 | V | BMI | 7 |
| Kim, 2010 [22] | Korea | 15 | 80 | 34.6±6.7 | 27.4+6.6 | NA | NA | 47.41 +33.66* | G | Gender, BMI | 8 |
Values are presented as number and mean±standard deviation. SLE: systemic lupus erythematosus, BMI: body mass index, NA: not available, A: adiponectin, V: visfatin, G: ghrelin, USA: United States of America.
Table 2.
Meta-analysis of the adiponectin levels in patients with SLE compared to healthy controls
| Group | Population | Number of study | Test of association | Test of heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| SMD* | 95% CI | p-value | Model | p-value | I2 | |||
| All | Overall | 12 | 0.360 | 0.025∼0.695 | 0.035 | R | 0.000 | 88.1 |
| Region | Western | 9 | 0.225 | 0.024∼0.426 | 0.028 | R | 0.018 | 56.6 |
| South American | 2 | 0.746 | −1.571∼3.062 | 0.528 | R | 0.000 | 98.2 | |
| Asian | 1 | 0.993 | 0.583∼1.403 | 2.1×10−6 | NA | NA | NA | |
| Matched variables† | Yes | 9 | 0.273 | 0.015∼0.531 | 0.008 | R | 0.000 | 71.4 |
| No | 3 | 0.632 | −0.808∼2.072 | 0.390 | R | 0.000 | 95.6 | |
| Sample size, n | <100 | 4 | 0.082 | −0.386∼0.550 | 0.731 | R | 0.010 | 73.3 |
| ≥100 | 8 | 0.492 | 0.065∼0.920 | 0.024 | R | 0.000 | 90.6 | |
| Mean age, yr | <40 | 6 | 0.307 | −0.336∼0.950 | 0.349 | R | 0.000 | 93.0 |
| ≥40 | 6 | 0.429 | 0.124∼0.733 | 0.006 | R | 0.003 | 72.1 | |
Table 3.
Meta-analysis of visfatin and ghrelin levels in patients with SLE compared to healthy controls
| Group | Population | Number of study | Test of association | Test of heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| SMD* | 95% CI | p-value | Model | p-value | I2 | |||
| Visfatin | Overall | 3 | 0.451 | −0.000∼0.903 | 0.050 | R | 0.018 | 75.2 |
| Western | 1 | 0.365 | 0.072∼0.658 | 0.015 | NA | NA | NA | |
| South American | 1 | 0.892 | 0.517∼1.267 | 3.2×10−6 | NA | NA | NA | |
| Middle Eastern | 1 | 0.025 | −0.505∼0.554 | 0.927 | NA | NA | NA | |
| Ghrelin | Overall | 4 | 0.611 | −0.383∼1.605 | 0.228 | R | 0.000 | 94.3 |
| Western | 2 | 0.560 | 0.258∼0.861 | 2.7×10−4 | F | 0.170 | 46.9 | |
| South American | 1 | 2.000 | 1.562∼2.438 | <1.0×10−8 | NA | NA | NA | |
| Asian | 1 | −0.478 | −1.034∼0.077 | 0.091 | NA | NA | NA | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download