Abstract
Brown adipose tissue (BAT) is related with energy expenditure, in contrary to fat-storing white adipose tissue. Recent studies have shown that cold exposure could be related with the expression of BAT in adult subjects assessed by 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET). In addition, the application in previous clinical trials showed positive effect of xanthigen containing fucoxanthin and punicic acid on body weight and liver fat content. In this short-term intervention study, we evaluated the effect of xanthigen on the expression of BAT by 18F-FDG PET. Two healthy obese premenopausal women were enrolled and xanthigen 600 mg (2 capsules including fucoxanthin 3 mg, punicic acid 174 mg) was given for 3 months without dietary and exercise intervention. Body composition and dietary intake were assessed monthly. Laboratory test and 18F-FDG PET were performed before and after intervention. After intervention, there was neither weight reduction nor remarkable laboratory change. However, BAT, assessed by 18F-FDG PET, was detected in both cervical, supraclavicular and paravertebral space in one subject, even though her body weight showed mild increase. This result suggested that xanthigen can induce BAT in a healthy adult. However, a further large well-controlled study is needed.
Early investigation12 showed that brown adipose tissue (BAT) was observed in subjects during cold exposure, assessed by 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET). Furthermoere, fucoxanthin from edible seaweed, Undaria pinnatifida, exhibited antiobesity effect through UCP1 expression in white adipose tissues,3 and a clinical trial using xanthigen containing fucoxanthin and punicic acid showed positive effects on weight loss, body fat and liver fat content in obese non-diabetic women. In the present study, therefore, we conducted a pilot study for application and evaluation of short term effect of xanthigen on BAT expression in two healthy obese premenopausal women and the results described.
Two premenopausal healthy obese women with body mass index >30 kg/m2 were voluntarily enrolled in this pilot study after informed written consent. They were enrolled in the mid August and the study was conducted until mid November. After the enrollment, the subjects were requested to visit our department every month for measurement of body weight and body composition, and dietary record during the 3 month intervention period. For 3 months, xanthigen 600 mg (2 capsules including fucoxanthin 3 mg and punicic acid 174 mg) was given to the study subjects. They were instructed to take xanthigen before going to bed and not to change their regular lifestyle such as exercise or physical activity and dietary intake. The effect of xanthigen on the expression of BAT, as well as the changes of body weight and metabolic markers were evaluated. Institutional Review Board in Ajou University of Hospital approved the study (AJIRB-MED-FOD-14-196). Body composition was measured by dual-energy X-ray absorptiometry (DXA, DISCOVERY-W fan-beam densitometer, Hologic Inc., Malborough, MA, USA). We assessed three day dietary recall before intervention and every scheduled visit during the study period. Subjects were asked to fast and prohibited from parenteral infusion of sugar-containing fluids at least 6 hours before 18F-FDG PET scanning. They were also asked not to undertake strenuous exercise for 1 day before examination. Blood glucose concentration was measured immediately before an intravenous administration of 18F-FDG to ensure a level below 150 mg/dL. After an injection of 370 MBq 18F-FDG, the subjects were instructed to rest comfortably for 60 minutes and to empty the bladder just before scanning. All laboratory tests and 18F-FDG PET were conducted before and after intervention.
The results indicated no weight reduction by this intervention trial. Small sample size makes it difficult to clearly confirm the effect of xanthigen (Table 1). Nevertheless, we assessed regional BAT by 18F-FDG PET in both subjects before and after intervention. Regional measured areas included the neck, shoulders, and clavicles. Interestingly, after 12 week intervention, regional BAT in one subject (A) was detected, as compared to baseline examination. In the other subject (B), however, there was no significant focal FDG uptake, suggesting BAT before and after intervention (Fig. 1).
In this small pilot study, we identified the expression of BAT by 18F-FDG PET after 3 months of xanthigen application, even though there was no weight reduction, indicating that xanthigen containing fucoxanthin and punicic acid can induce BAT in adult adipose tissues. A previous study already demonstrated the positive effect of xanthigen on body weight and liver fat due by increasing resting metabolic rate.4 Fucoxanthin can induce UCP1 in adipose tissue3 and can be expressed as a form of beige adipose tissue. Beige cells resemble white cells with extremely low basal expression of UCP1, but like classical brown fat, they respond to cyclic AMP stimulation with high UCP1 expression and respiration rates.5 However, another study showed that all human BAT abundantly expressed beige cell-selective genes, but the expression of classical brown fat-selective genes were nearly undetectable.6 There seems to be an important physiological cross-talk between the constitutive (BAT) and recruitable (beige) brown fat cells.7
The sympathetic nervous system is currently seen as the main effector for brown fat function8 and white adipocytes of pure white fat that are subject to adrenergic stimulation are able to undergo a process of direct transformation into brown adipocytes. 9 Under cold exposure, the sympathetic nerve system is activated via the expression of cold receptor TRPM8 in human adipocytes inducing their "browning". This suggests a possible role of this receptor in the control of adipose tissue metabolism and body energy balance.10 Likewise, therapeutic effects can be expected from the use of specific drugs or food compounds such as xanthigen that are able to induce a program of brown fat differentiation, including UCP1 expression and enhanced oxidative metabolism in white adipose cells.311 In animal studies, dietary fucoxanthin was found to accumulate in the heart and liver as fucoxanthinol and as amarouciaxanthin A in adipose tissue;12 their metabolites were confirmed.13 A recent review article highlighted the effect of fucoxanthin on obesity, metabolic syndrome, and diabetes therapy,1415 as well as many other BAT activating factors.16
This study has some limitations. First, the confounding factors such as cold exposure or total calorie intake changes were not well controlled. Second, we could not confirm the causal-relationship of xanthigen application and BAT expression by such short-duration and small case report study. Long-term intervention trial is needed.
Nevertheless, this is the first study to evaluate BAT expression, by using 18F-FDG PET in the adult human, after xanthigen application for 3 months. Furthermore, we clearly confirmed the BAT in one subject after intervention, supporting the previous studies that showed a positive effect of xanthigen on the increase of energy expenditure and BAT induction in obese subjects. Nevertheless, a large intervention study is needed in future.
Figures and Tables
Fig. 1
Brown adipose tissue was assessed by 18F-fluorodeoxyglucose (FDG) positron emission tomography-CT before and after intervention. In subject A (above 4 pictures), multifocal brown fat uptake in both cervical, supraclavicular and paravertebral space was noticed after 3 months xanthigen intervention. In subject B (below 4 pictures), however, there was no demonstrable focal 18F-FDG uptake to suggest brown fat.
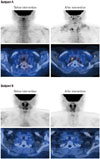
Table 1
Changes of Body Composition, Metabolic Parameters before and after Intervention

Bwt, body weight; BMI, body mass index; Fat mass, total body fat mass; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TSH, thyroid stimulating hormone; FT4, free thyroid; hsCRP, high-senstive C-reactive protein; FFA, free fatty acid; Total calorie, daily total energy intake.
ACKNOWLEDGEMENTS
We specially thank Bokkee Min, Seongho Yun, and Sukyung Kwon, who are managers of NOVAREX Company. They generously funded for this pilot study and also supplied xanthigen in the study. We also thank Professor Joon-Kee Yoon, Department of Nuclear Medicine and Molecular Imaging, Ajou University Hospital for measurement of BAT by 18F-FDG PET.
References
1. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009; 360:1500–1508.

2. Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring). 2011; 19:13–16.

3. Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun. 2005; 332:392–397.

4. Abidov M, Ramazanov Z, Seifulla R, Grachev S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes Metab. 2010; 12:72–81.

5. Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012; 150:366–376.

6. Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012; 7:e49452.

7. Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013; 495:379–383.

8. Broeders E, Bouvy ND, van Marken. Endogenous ways to stimulate brown adipose tissue in humans. Ann Med. 2015; 47:123–132.

9. Frontini A, Vitali A, Perugini J, Murano I, Romiti C, Ricquier D, et al. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim Biophys Acta. 2013; 1831:950–959.

10. Rossato M, Granzotto M, Macchi V, Porzionato A, Petrelli L, Calcagno A, et al. Human white adipocytes express the cold receptor TRPM8 which activation induces UCP1 expression, mitochondrial activation and heat production. Mol Cell Endocrinol. 2014; 383:137–146.

11. Bonet ML, Oliver P, Palou A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta. 2013; 1831:969–985.

12. Hashimoto T, Ozaki Y, Taminato M, Das SK, Mizuno M, Yoshimura K, et al. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br J Nutr. 2009; 102:242–248.

13. Sangeetha RK, Bhaskar N, Divakar S, Baskaran V. Bioavailability and metabolism of fucoxanthin in rats: structural characterization of metabolites by LC-MS (APCI). Mol Cell Biochem. 2010; 333:299–310.

14. Maeda H. Nutraceutical effects of fucoxanthin for obesity and diabetes therapy: a review. J Oleo Sci. 2015; 64:125–132.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download