Abstract
Critical limb ischemia (CLI) is one of the most severe forms of peripheral artery diseases, but current treatment strategies do not guarantee complete recovery of vascular blood flow or reduce the risk of mortality. Recently, human bone marrow derived mesenchymal stem cells (MSCs) have been reported to have a paracrine influence on angiogenesis in several ischemic diseases. However, little evidence is available regarding optimal cell doses and injection frequencies. Thus, the authors undertook this study to investigate the effects of cell dose and injection frequency on cell survival and paracrine effects. MSCs were injected at 106 or 105 per injection (high and low doses) either once (single injection) or once in two consecutive weeks (double injection) into ischemic legs. Mice were sacrificed 4 weeks after first injection. Angiogenic effects were confirmed in vitro and in vivo, and M2 macrophage infiltration into ischemic tissues and rates of limb salvage were documented. MSCs were found to induce angiogenesis through a paracrine effect in vitro, and were found to survive in ischemic muscle for up to 4 weeks dependent on cell dose and injection frequency. In addition, double high dose and low dose of MSC injections increased vessel formation, and decreased fibrosis volumes and apoptotic cell numbers, whereas a single high dose did not. Our results showed MSCs protect against ischemic injury in a paracrine manner, and suggest that increasing injection frequency is more important than MSC dosage for the treatment CLI.
Critical limb ischemia (CLI) is one of the most severe forms of peripheral artery disease (PAD). Patients with CLI suffer not only from severe pain but also from paleness, pulselessness, and/or paralysis of affected legs. Among those patients with non-recoverable CLI symptoms, about 40% eventually undergo leg amputation, and around 20% die within 6 months of presentation [1].
The risk factors for CLI include diabetes mellitus (DM), hyperlipidemia, age, and smoking [1]. These factors can cause minor or major thromboses inside blood vessels and result in ischemic conditions. Thrombosis is defined the localized clotting of blood in the circulatory system, and since these clots remain in blood vessels, they impede blood flow. Furthermore, when present in a femoral artery, nutrient and oxygen supply are reduced and critical limb ischemia can result [2]. As well as acting as a physical obstruction, atherosclerosis induces increases in the plasma levels of inflammatory proteins, such as, interleukin 6 (IL-6), neopterin, and tumor necrosis factor-alpha (TNF-α), and these up-regulations are associated with 1-year mortality despite clinical intervention [3].
The clinical strategies commonly used to prevent limb loss in CLI target the restoration of blood supply and include mechanical or pharmacological procedures or surgery [4,5,6]. However, these efforts do not guarantee complete or permanent recovery of vascular blood flow and have little effect on patient survival.
Recently, mesenchymal stem cells (MSCs) injections have been used to overcome post-ischemic injuries [7]. MSCs have been reported to have positive effects on angiogenesis via paracrine secretion in patients with ischemic injuries, such as, myocardial infarction (MI) [8] or brain stroke [9].
It is well known MSCs secrete angiogenic related proteins, such as, vascular endothelial growth factor (VEGF) and angiopoietin 1 (ANG-1) in vitro and in vivo [10,11]. These proteins are required for blood vessel formation [12,13,14] via the synthesis of extracellular matrix [15], migration, endothelial cell proliferation [16], angiogenesis, and vessel growth [17] in the presence of ischemic disease [16]. In addition, to the direct angiogenic effect of MSCs, several recent studies have shown that various proteins secreted by MSCs stimulate M2 macrophage differentiation [18,19]. M2 macrophages uniquely increase the productions of endothelial cells and tubular structures and express related genes, such as, insulin-like growth factor-1 (IGF1), chemokine ligand 2 (CCL2), basic fibroblast growth factor (bFGF2), and placental growth factor (PLGF) [20]. These proteins are activated by pro-enzyme of matrix metalloproteinase-9, which is produced by infiltrating macrophages [21,22].
Potentially, MSCs administration offers a powerful means of treating CLI. Several clinical trials have been conducted on the use of MSCs from both allogeneic and autologous tissues and other trials are on-going [23]. Furthermore, several preclinical studies are being conducted to optimize MSCs treatments with respect to dosage, injection frequency, injection route, and injection site in animal models of CLI [23]. Cell dose and injection frequency are crucial in animal models and in CLI patients. When MSCs are injected into CLI animal models, direct intramuscular injection and multiple injections (3 to 5 injections) are preferred in ischemic regions [23]. Preclinical studies have demonstrated that MSCs are therapeutically effective in CLI animal studies and that they appear to have a satisfactory safety profile.
Although cell dose and injection frequency are important, little information is available on the topic in animal models of CLI. Accordingly, we sought to determine whether injected MSCs dose and frequency influence muscle status or improve MSCs survival and how they affect angiogenic factor secretion and polarize M2 macrophages in a murine model of CLI.
Human bone marrow derived mesenchymal stem cells (MSCs) and human umbilical vein endothelial cells (HUVECs) were used in this study. MSCs were cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, NY, USA) containing a low glucose concentration supplemented with 10% fetal bovine serum (FBS; Life Technologies) and 1% gentamycin. HUVECs were grown in EGM™-2 growth medium (Lonza Biosciences, ML, USA) in 1% gelatin coated culture dishes. All cells were maintained in 5% CO2 humidified incubator at 37℃.
Adult (7 week-old) male Balb/c mice (weight 21~26 gm) were maintained in a temperature-controlled room with access to food and water ad libitum. All experiments were approved by the Institute Animal Care and Use Committee of Lee Gil Ya Cancer and Diabetes Institute of Gachon University and were conducted humanely (approval number; LCDI-2013-0002). Mice were anesthetized with Zoletil (25 mg/kg) and Rompun (5~10 mg/kg) by intra-peritoneal injection. After anesthesia induction, thrombus of the left femoral artery was induced by topically applying a small strip of filter paper soaked with 10% ferric chloride (Sigma-Aldrich, MO, USA) for 3 mins. After 3 mins, MSCs (106 or 105 cells; high or low doses) were mixed with 80 µl of Plasma Solution A (CJ Cheil Jedang Corp., South Korea), and then injected into the ischemic skeletal muscle area in the single injection model. For control model, the same volume (80 µl) of Plasma Solution A (saline) was intramuscularly injected into ischemic areas. In the double injection model, MSCs or saline were injected once in 2 consecutive weeks in the same ischemic area. Protein and mRNA samples of skeletal muscles were obtained 4 weeks after MSCs first implantation.
After antigen retrieval using sodium citrate solution (ph. 7.2), tissue sections were incubated in blocking solution with normal horse serum to block non-specific binding. The tissues were incubated with specific antibodies (detailed in Supplemental Table 1) overnight at 4℃ and rinsed with phosphate buffer saline (PBS, Life technologies). The tissues were incubated with fluorescence conjugated secondary antibodies for 1 hr (Supplemental Table 1) rewashed with PBS. Nuclei were counterstained with 4', 6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich) at room temperature for 10 mins. Fluorescence signals were detected using a confocal microscope (LSM 710, Carl Zeiss) and fluorescence intensities and blood vessel sizes were measured using Zeiss Zen 2012 software (Carl Zeiss).
MSCs were supplied by FCB-Pharmicell (Sungnam, South Korea). These cells were extracted from healthy bone marrow of 51 years old man and passaged 5~6 times. To confirm their identities, the well-known MSCs specific stem cell markers, CD73 (+), CD105 (+), CD34 (–), and CD45 (–), were checked by fluorescence-activated cell sorting (FACS) analysis. The MSCs differentiated into adipocytes (adipogenic differentiation) or osteocytes (osteogenic differentiation) for confirmation of multipotent and each MSCs derived cells were especially stained with Oil Red O or alkaline phosphatase respectively.
MSCs (2×105 cells) were labeled with CD105, CD34, and CD45 conjugated with Fluorescein isothiocyanate (FITC) and CD73 conjugated with phycoerythrin (PE) for 20 mins at room temperature in the dark. To check negative control, isotype controls was labeled with same FITC and PE. After incubation with antibodies, washed with PBS and stained cells were measured with FACS (Navios™ flow cytometer, Beckman Coultyer) and data analyzed by kaluza software (Beckman Coultyer).
To check the osteogenic differentiation ability, MSCs were incubated with osteogenic differentiation medium which is mixed with 0.1 µM dexamethasone (Sigma-Aldrich), 10 mM β-glycerol phosphate (Sigma-Aldrich) and 50 µM L-ascorbic acid (Sigma-Aldrich) in MSCs growth medium including 10% FBS, 100 U/mL penicillin (Life Technologies), 100 mg/mL streptomycin (Life Technologies), high-glucose DMEM (Life Technologies) for 14 days at 37℃ and 5% CO2. MSCs (3×105) were planted into 6-well plate (BD Falcon) and the osteogenic differentiation medium was changed every third day. After PBS washed, differentiated cells were fixed with 100% methanol (Merck Millipore, Ohio, USA) in ice for 2 mins and washed with distilled water twice. The cells incubated with alkaline phosphatase substrate, 5-Bromo-4-Chloro-3-Indolyl Phosphate / Nitroblue Tetrazolium (BCIP/NBT) liquid substrate (Sigma-Aldrich), for 10 mins at room temperature. For validation of adipogenic differentiation potency, same number of MSCs was used. Adipogenic differentiation induction medium is prepared with 1 µM dexamethasone, 10 µg/mL insulin, 100 µM indomethacin, and 0.5 mM methyl isobutylzanthine in MSCs growth medium for 72 hrs. After PBS washed twice, differentiated cells were fixed with 4% paraformaldehyde for 4 hrs and stained with 60% oil red-O solution (Sigma-Aldrich) for 45 mins at room temperature. Used reagents for adipogenic differentiation were obtained from Sigma-Aldrich. All differentiated cells were observed by light microscope (Olympus CX21).
To determine the collagen formation in the ischemia area, we have performed Masson's Trichrome staining by using commercial kit (Sigma-Aldrich). To fix the skeletal muscle tissues slides, put slides in the bouin's solution (Sigma-Aldrich) for 15 mins at 56℃. Afterwards, tissues were stained with iron hematoxylin solution (Merck Millipore) for 5 mins to observe nucleus, then Biebrich Scarlet solution (Sigma-Aldrich) was used for 2 mins to stain acidophilic tissue components. These slides were treated with phospho-acids, phosphomolybdic-phosphotungstic acid solution (Sigma-Aldrich), for differentiating tissues. Lastly, tissues slides were washed with running water and collagen of skeletal muscle tissues slides was stained by aniline blue solution (Sigma-Aldrich).
All stained tissues slides were immersed in each absolute alcohol and xylene for 3 mins and these slides were covered with glass by using DPX mounting medium (Sigma-Aldrich) and they were analyzed under the light microscope.
To detect apoptotic cells after ferric chloride treatments, TUNEL was performed according to the instruction. Briefly, tissues sections were incubated with mixture of TUNEL reagent for 1 hr at 37℃ in the dark. Nuclei were counterstained by DAPI. After PBS wash, tissue sections were mounted with Vectashield mounting medium and coverslipped on glass slide and validated by confocal microscope.
Total RNA in MSCs or saline injected muscles was isolated using the Trizol methods following to the provided manufacture's manual. The muscle tissues were homogenized on ice using a disposable pestle in 1 ml Trizol reagent (Invitrogen, CA, USA). Homogenized samples were mixed with 0.2 ml chloroform (Amresco, OH, USA). After the sample was centrifuged at 12,000 × g for 15 mins at 4℃, the aqueous phase was collected into a new tube, mixed with 0.5 ml absolute isopropanol, leaving only the RNA pellet. To clarify the RNA, isolated RNA was washed with 70% ethanol. The dried pellet was dissolved with 30 µl DEPC water.
The muscle tissues were fixed in 4% paraformaldehyde in PBS, and then followed by soaking in 20% sucrose solution overnight at 4℃ for cryoprotecion. To make muscle tissue frozen block, muscles were frozen in isopentane cooled by liquid nitrogen. The cryoprotected muscle tissues were cut into 10 µm slices by using a cryotome (Leica, Wechsler).
Sectioned tissue slides were stained followed by immunofluorescence staining. Diameter of vWF positive blood vessels were measured by Zen 2009 software (Carl zeiss, Germany).
To investigate proteins secreted by MSCs, we measured angiopoietin-1 (ANG-1), hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) and in mesenchymal stem cells-conditioned medium (MSC-CM). Briefly, MSC-CM was incubated in 96-well plates at 37℃ for 90 mins and then each primary antibody for 2 hrs at room temperature and then washed with PBS containing 0.1% Tween 20 (Sigma-Aldrich). Secondary antibodies were administered for 2 hrs at room temperature. After developing with tetramethylbenzidine (TMB, Sigma-Aldrich) solution for 10 mins in the dark and stopping the reaction with 2 N H2SO4 (Sigma-Aldrich), absorbances were measured at 450 nm using a spectrophotometer.
To confirm the angiogenic capacity of MSCs, we prepared MSC-CM and implemented angiogenesis assays. When we used HUVECs for this study, all HUVECs starved by incubating them for 24 hrs in HUVECs basal medium which's components is growth medium containing 1% FBS without growth factors. MSCs was incubated for 2 days in normal cell culture condition and then, this MSC-CM was concentrated by Amicon Ultra Centrifusal Filter (Merck Millipore).
Wound migration assay was used for validation of chemotactic motility. A scratch wound of 100% confluent HUVECs was introduced with a sterile pipette tip, and treating cell with PBS or MSC-CM. After incubation for 20 hrs, numbers of cells that migrated into wound fields were counted using the Image J program (NIH).
Tube formation was estimated using a matrigel assay. In brief, growth factor-reduced matrigel (BD Biosciences, San Diego, USA) was spread onto a 4-well plate (BD Falcon) and incubated for 40 mins at 37℃. After HUVECs starvation, HUVECs were seeded (5×104 cells/well) on the matrigel and then, treated with PBS or MSC-CM. After 20 hrs incubation, images were captured by light inverted microscope (Olympus CKX31).
Endothelial cells proliferation was determined using a tetrazolium / phenazine methosulfate (MTS/PMS, Promega) assay. Briefly, HUVECs (1×104 cells/well) seeded in a 96-well plate (BD Falcon) for FBS starvation. Cells were then treated with PBS or MSC-CM at 37℃ for 48 hrs. Freshly prepared MTS/PMS solution (20 µl/well) was mixed HUVECs basal medium (100 µl/well) in the plates and then cells were incubated for 4 hrs at 37℃ in the dark. Absorbance was read at 490 nm using a spectrophotometer (PerkinElmer). All experiments were performed three times in triplicate.
HUVECs were incubated in basal medium (Basal-M) or MSC-CM for 2 days. Whole cell lysates containing equal amount of proteins (30 µg protein/lane) were resolved by 4~12% NuPAGE Bis-Tris gel (Thermo Scientific, NH, USA) and transferred to polyvinylidene fluoride (PVDF) membrane using semi-dry (25 voltage, 10 mins; ATTO, Japan). For protein isolation, cells were collected in RIPA buffer (TAKARA, Japan) with protease inhibitor and phosphatase inhibitors (TAKARA). The PVDF membranes were incubated with 5% skim milk as a blocking solution, followed by proper primary antibodies (Supplemental Table 1) in blocking solution overnight at 4℃.
The membranes were washed with Tris-buffered saline with 0.1% tween 20 (TTBS) three times and incubated with appropriate secondary antibodies (Supplemental Table 1) for 1 hr at room temperature. The blotting membranes were developed with enhanced chemiluminescence (ATTO) on LAS-4000 (GE healthcare).
To validate injected MSCs viability, human specific markers including CD29 and CD44 genes were used. After RNA extraction, complementary DNA (cDNA) was synthesized by using the PrimeScript 1st strand cDNA Synthesis Kit (TAKARA) followed by manufacturer's manual. One hundred nano gram of each cDNA, 5 µl SYBR Premix (TAKARA) and 0.4 µM forward and same volume of reverse primers (listed in Supplemental Table 2) were mixed, then the level of interesting gene expression was detected on a CFX384 Touch™ Real-Time PCR Detection System (Bio-Rad, CA, USA).
p-values were calculated and analyzed using SPSS software version 22 (SPSS, IL, USA). The Kruskal-Wallis test and the Mann-Whitney U test were used to determine the significances of differences between MSCs viability, blood vessel sizes, M2 macrophage infiltrations, and histology findings. p-values of <0.05 were considered significant.
Extracted MSCs showed typical surface markers and differentiation potentials. Analysis of MSCs surface markers by FACS revealed 99.99% and 99.98% were CD73 or CD105 positive, respectively, and that 0.78% and 0.63% were CD34 or CD45 positive (Supplemental Fig. 1A). MSCs also differentiated into adipocytes or osteocytes depending on the differentiation method used. To assess adipogenesis of MSCs, lipid droplets were stained with Oil Red O.
Differentiated osteocytes showed positivity to alkaline phosphatase, in areas of mineral deposition (Supplemental Fig. 1B). To assess the effects of injection frequency and of cell dose on MSCs survival, we analyzed protein and mRNA levels of injected MSCs in ischemic areas. CD44 levels were confirmed by immunofluorescence analysis. Double high dose of MSCs injection in ischemic muscles resulted in the largest number of CD44 expressing cells, and double low dose of MSCs injection resulted in 1.59 fold increase in the number of CD44 expressing MSCs than a single high dose of MSCs injection (p<0.05; Fig. 1A). qRT-PCR results for CD29 and CD44 showed that the survival rate of injected MSCs resulting from double high dose of MSCs injection was greatest. Interestingly, expression level of CD29 in double low dose injected tissues was 2.33-fold higher than those of single high dose injected tissues. CD44 expression pattern was similar with CD29. The level of CD44 in double low dose of MSCs injections was 1.69-fold higher than in single high dose of MSCs injection (p<0.05; Fig. 1B). Based on these results, double injections of either high or low dose of MSCs provided better cell survival than a single high dose injection.
To investigate ability of MSCs to induce angiogenesis, we measured the migration, tube formation, and proliferation ability in HUVECs. Mean migration ability and mean tube length formed by HUVECs after MSC-CM treatment were 9.30-fold (Fig. 2A) and 3.89-fold higher (Fig. 2B), respectively, than in PBS. Moreover, HUVECs proliferation induced by MSC-CM was 2.28-fold greater than that induced by PBS alone (Fig. 2C). ANG-1, HGF, and VEGF are important growth factors during angiogenesis, and we used ELISA to assess levels of ANG-1, HGF, and VEGF proteins excreted into MSC-CM. ELISA results showed that amounts of secreted ANG-1, HGF and VEGF were 1.77, 1.98, and 1.59-fold higher, respectively, in MSC-CM than in basal medium (Fig. 2D). To study the angiogenesis mechanism in vitro, we investigated changes in the expressions of pAKT and MAPK in MSC-CM treated HUVECs. Immunoblot analysis showed MSC-CM treatment increased pERK1/2 and pAKT expressions. However, the expression levels of AKT, ERK1/2, p38, pp38, SAPK/JNK, and pSAPK/JNK did not change after MSC-CM treatment (Fig. 2E). In vivo, injected MSCs were found to promote angiogenesis. The size of vWF positive blood vessels after double high dose of MSCs injections were significantly greater (by 2.63 and 3.25-fold) than single high dose or double low dose of MSCs injection (Fig. 2F). Interestingly, no significant differences were observed between vWF positive vessel sizes after double low dose injections or single high dose of MSCs injection, and single low dose injection resulted in larger vessels that single high dose of MSCs injection. These results indicate that injection frequency is more important than the dose administered with respect to the promotion of angiogenesis by MSCs in limb ischemia.
In order to confirm the effect of MSCs on M2 macrophage modulation, we used immunofluorescence analysis and a M2 specific marker arginase 1 protein (Arg 1). The number of Iba1 positive cells (a marker of macrophage activation) was higher in saline injected mice than in MSCs treated mice. However, it was changed in all MSCs injected mice except after a single low dose injection and these difference among high dose of MSCs and double low dose of MSCs injection was not stand out (Fig. 3A, B).
Although the number of Iba1 positive cells decreased after MSCs injection, the proportion of Arg 1 expressing Iba1 cells was increased by MSCs injection. In particular, the number of Arg 1 expressing Iba1 cells was significantly higher after single and double high dose of MSCs injections than after double low dose injections (Fig. 3B). These results show the higher MSCs doses than minimum number (105 MSCs) of MSCs better modulated macrophage and M2 macrophage infiltrations than increasing injection frequency.
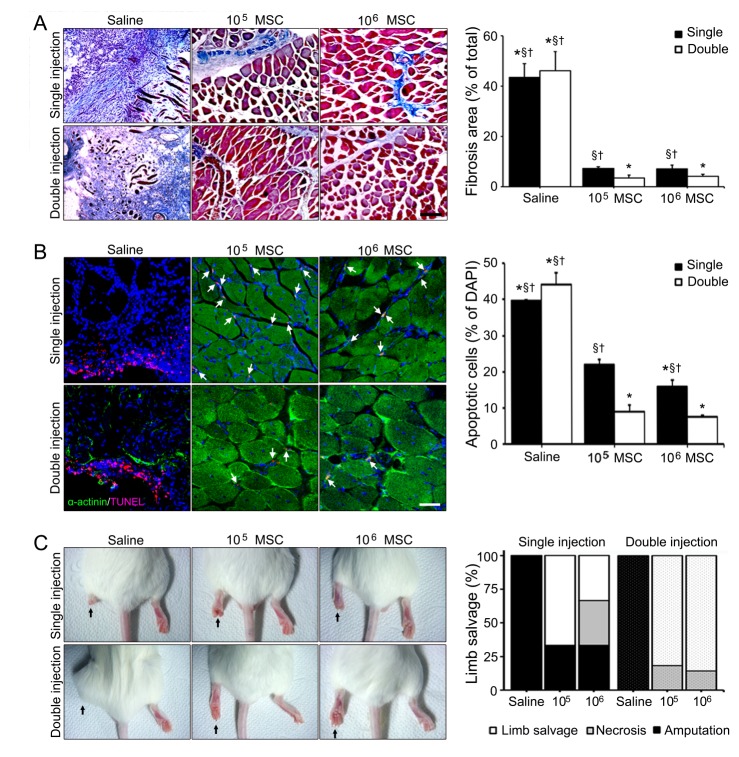
Limb ischemia induced fibrosis and apoptosis were dramatically reduced in 4 weeks after single or double high dose of MSCs injections, but not after single low dose of MSCs injection (Fig. 4A, B). Double high and low dose of MSCs injections reduced fibrosis areas by 12.92 and 10.97-fold, respectively, versus double injections of saline (Fig. 4A). Numbers of apoptotic cells (as indicated by TUNEL staining) were reduced by 4.09, 5.95-fold after double high or low dose of MSCs injections, respectively, versus double injections of saline (Fig. 4B). Interestingly, no statistical difference was observed between animals administered double high or low dose of MSCs injections.
Finally, limb conditions in all MSCs injected groups were better than in the saline group (Fig. 4C). Among the all MSCs injected groups, limb salvage ratio was highest in the double high dose of MSCs injection group (85.71%) followed by the double low dose of MSCs injection group (81.82%), which both had greater limb salvage ratios than the single high or low dose of MSCs injection groups. Necrosis ratio in the double high dose of MSCs injection group was lowest (14.29%) and the double low dose of MSCs injection group was next lowest (18.18%). These results indicate injection frequency is more important than dosage in terms of preventing limb loss in limb ischemia (Fig. 4C).
MSCs are usually obtained from bone marrow [24], adipose tissues [25], or umbilical cord blood [26], and are characterized by several factors, such as, morphology, surface markers, and stemness [27]. They have a morphology similar to that of slender fibroblasts and they grow attached in culture. CD44, CD73, CD90 and CD 105 are positive cell-surface markers for MSCs and CD45 (known as leucocyte common antigen) and CD34 (known as hematopoietic stem marker) are negative markers [28,29]. MSCs can differentiate into mesodermal cells, such as, adipocytes, chondrocytes, and osteocytes [27]. In the present study, MSCs exhibited typical characteristics with respect to cell surface markers and differentiation into adipocytes and osteocytes (Supplemental Fig. 1).
Paracrine effects of MSCs therapy are of central importance, and thus, in view of their paracrine effects the survival of injected MSCs is considered an issue of key concern. When we checked survival rate of injected cells in our CLI animal model for 4 weeks after first injections, we found it depended on cell dose and injection frequency. Our results showed that the survival rate after double high dose of MSCs injections was greatest followed by double low dose of MSCs injections. Furthermore, the survival rate after a single high dose of MSCs injection was lower than after double low dose of MSCs injections (Fig. 1), which means that injection frequency needs to be considered as well as dosage when considering MSCs therapy in CLI.
Our results show MSCs have a strong paracrine effect on CLI in mice (Fig. 2). Angiogenic or growth factors secreted by MSCs caused endothelial cells to increase, migrate, and form tubes in vitro. Furthermore, MSCs secrete several types of cytokines and chemokines, such as, ANG-1, IL-6, monocyte chemoattractant protein-1 (MCP-1), and VEGF [30]. Because of these paracrine functions of angiogenic factors, attempts have been made to use MSCs-based cell therapies to treat ischemic diseases, such as, ischemic stroke, myocardial infarction (MI), and CLI [31,32,33]. Clinical trials on CLI patients [34] have shown MSCs treatment improved collateral vessel formation in a diabetic mellitus patient with a foot ulcer [35] and in a patient with Buerger's disease [36].
It has been proposed that the mechanism responsible for the effects of MSCs therapy involves paracrine or autocrine effects, as MSCs secrete many types of cytokines and chemokines [37]. Our results show MSCs treatment increased arteriogenesis and angiogenesis by operating at a molecular level. The classical angiogenic factors, ANG-1, HGF, and VEGF proteins are increased in MSC-CM treated endothelial cells. Furthermore, these angiogenic factors may activate AKT and ERK1/2, which are essential for angiogenesis [10]. Our results showed that MSC-CM induced the activations of AKT pathway and ERK1/2 pathway, but not the activations of p38 or SAPK/JNK in HUVECs, and induced the angiogenesis of endothelial cells. Secreted molecules like ANG-1, HGF, and VEGF are required for blood vessel formation [12,13,14] via the synthesis of extracellular matrix [15], endothelial cell migration, endothelial cell proliferation [16], angiogenesis, and vessel growth [17] in the presence of ischemic disease [16]. Furthermore, the PI3K-AKT pathway modulates the functions of endothelial cells and promotes angiogenesis whereas ERK induces cell proliferation [38].
A recent study showed MSCs regulate M1 and M2 balance in a MI animal model [39]. In MSCs injected myocardium, adjacent macrophages strongly expressed Arg 1, and in an in vivo study, MSCs co-cultured macrophages expressed fewer M1 markers including interleukin-6 (IL-6), IL-1β, MCP-1, and inducible nitric oxide synthase (iNOS) than control group. In contrast, M2 markers, including, IL-4, CD206, IL-10, and Arg1 were noticeably increased by co-culturing macrophages with MSCs [39]. MSCs are also key modulators of M2 macrophages in CLI [40]. Several researchers have reported that have demonstrated a more prominent role of M2 macrophages in arteriogenesis. M2 macrophages express a considerable number of angiogenic growth factors, such as, VEGF-A, TGFα, IGF-1, PDGF-β and HGF, and promote wound repair and neovascularization [41,42,43]. Moreover, a study showed tube formation was performed by the CM of M2 macrophages incubation, whereas the CM of M0 (rest condition) and M1 macrophages incubation actually inhibited tube formation, in vitro [12]. Thus, factors that induce the M2 phenotype can be considered to be indirectly angiogenesis by virtue of their ability to elicit angiogenic factor synthesis in and release from macrophages. Interestingly, our results show that high dose of MSCs injection better M2 macrophages than increasing treatment frequency.
Ischemia severity, as determined by ischemia scores and leg amputation rates, improved in the double MSCs injection groups at 4 weeks, in addition, blood vessel numbers were higher in these double MSCs injection groups.
In summary, our study shows injection frequencies and doses must be considered when planning MSCs therapy in patients with limb ischemia, and that increasing injection frequency enhances the survival of injected MSCs, which increase their paracrine effects.
ACKNOWLEDGEMENTS
We thank the FCB-Pharmicell company for providing the MSCs and Eak Kyun Shin for helpful comments of the manuscript. This study was supported by the Gachon University research fund (No. 2015-5102) and by the National Research Foundation of Korea Government (No. A121991 and 2015R1A2A2A01005212).
Notes
References
1. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Bell K, Caporusso J, Durand-Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E 3rd, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K. TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007; 33(Suppl 1):S1–S75. PMID: 17140820.

2. Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005; 115:3355–3362. PMID: 16322780.

3. Barani J, Nilsson JA, Mattiasson I, Lindblad B, Gottsäter A. Inflammatory mediators are associated with 1-year mortality in critical limb ischemia. J Vasc Surg. 2005; 42:75–80. PMID: 16012455.

4. Lyden SP. Endovascular treatment of acute limb ischemia: review of current plasminogen activators and mechanical thrombectomy devices. Perspect Vasc Surg Endovasc Ther. 2010; 22:219–222. PMID: 21411460.

5. Kingsnorth A, Bowley D. Fundamentals of surgical practice: a preparation guide for the intercollegiate MRCS examination. 3rd ed. Leiden: Cambridge University Press;2011.
6. Andaz S, Shields DA, Scurr JH, Smith PD. Thrombolysis in acute lower limb ischaemia. Eur J Vasc Surg. 1993; 7:595–603. PMID: 8270059.

7. Rosales OR, Mathewkutty S, Gnaim C. Drug eluting stents for below the knee lesions in patients with critical limb ischemia : longterm follow-up. Catheter Cardiovasc Interv. 2008; 72:112–115. PMID: 18412272.
8. Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, March KL. IFATS collection: human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009; 27:230–237. PMID: 18772313.

9. van Velthoven CT, Kavelaars A, Heijnen CJ. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatr Res. 2012; 71:474–481. PMID: 22430383.

10. Kawasaki K, Smith RS Jr, Hsieh CM, Sun J, Chao J, Liao JK. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003; 23:5726–5737. PMID: 12897144.

11. Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, Fiaccavento R, Carotenuto F, De Vito P, Baldini PM, Prat M, Di Nardo P. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006; 24:23–33. PMID: 16100005.

12. Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004; 94:678–685. PMID: 14739163.

13. Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, Yang YZ, Pan C, Ge J, Phillips MI. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004; 117:3–10. PMID: 14687695.

14. Onda T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab. 2008; 28:329–340. PMID: 17637706.

15. Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marbán L, Marbán E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012; 59:942–953. PMID: 22381431.

16. van Poll D, Parekkadan B, Borel Rinkes IHM, Tilles AW, Yarmush ML. Mesenchymal stem cell therapy for protection and repair of injured vital organs. Cell Mol Bioeng. 2008; 1:42–50.

17. Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008; 3:e1886. PMID: 18382669.

18. Selleri S, Bifsha P, Civini S, Pacelli C, Dieng MM, Lemieux W, Jin P, Bazin R, Patey N, Marincola FM, Moldovan F, Zaouter C, Trudeau LE, Benabdhalla B, Louis I, Beauséjour C, Stroncek D, Le Deist F, Haddad E. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget. 2016; 7:30193–30210. PMID: 27070086.

19. Yamada K, Uchiyama A, Uehara A, Perera B, Ogino S, Yokoyama Y, Takeuchi Y, Udey MC, Ishikawa O, Motegi S. MFG-E8 drives melanoma growth by stimulating mesenchymal stromal cell-induced angiogenesis and M2 polarization of tumor-associated macrophages. Cancer Res. 2016; 76:4283–4292. PMID: 27197197.

20. Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014; 17:109–118. PMID: 24013945.

21. Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000; 2:737–744. PMID: 11025665.

22. Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004; 114:623–633. PMID: 15343380.

23. Liew A, O'Brien T. Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther. 2012; 3:28. PMID: 22846185.

24. Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976; 4:267–274. PMID: 976387.
25. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–4295. PMID: 12475952.

26. Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989; 321:1174–1178. PMID: 2571931.

27. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. The International Society for Cellular Therapy position statement. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006; 8:315–317. PMID: 16923606.
28. Satterthwaite AB, Burn TC, Le Beau MM, Tenen DG. Structure of the gene encoding CD34, a human hematopoietic stem cell antigen. Genomics. 1992; 12:788–794. PMID: 1374051.

29. Thomas ML. The leukocyte common antigen family. Annu Rev Immunol. 1989; 7:339–369. PMID: 2523715.

30. Zhang P, Dong L, Yan K, Long H, Yang TT, Dong MQ, Zhou Y, Fan QY, Ma BA. CXCR4-mediated osteosarcoma growth and pulmonary metastasis is promoted by mesenchymal stem cells through VEGF. Oncol Rep. 2013; 30:1753–1761. PMID: 23863999.

31. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005; 57:874–882. PMID: 15929052.

32. Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004; 94:92–95. PMID: 15219514.

33. Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002; 360:427–435. PMID: 12241713.

34. Sultan S, Hynes N. Critical appraisal of stem cell therapy in peripheral arterial disease: Do current scientific breakthroughs offer true promise or false hope? J Biomed Sci Eng. 2014; 7:75–85.

35. Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011; 92:26–36. PMID: 21216483.

36. Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ, Park JH, Lee SY, Kim SP, Kim YD, Chung SW, Bae YC, Shin YB, Kim JI, Jung JS. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J. 2012; 76:1750–1760. PMID: 22498564.
37. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008; 103:1204–1219. PMID: 19028920.

38. Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006; 7:359–371. PMID: 16633338.

39. Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014; 46:e70. PMID: 24406319.

40. Richards J, Gabunia K, Kelemen SE, Kako F, Choi ET, Autieri MV. Interleukin-19 increases angiogenesis in ischemic hind limbs by direct effects on both endothelial cells and macrophage polarization. J Mol Cell Cardiol. 2015; 79:21–31. PMID: 25450612.

41. Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007; 204:1057–1069. PMID: 17485518.

42. Bréchot N, Gomez E, Bignon M, Khallou-Laschet J, Dussiot M, Cazes A, Alanio-Bréchot C, Durand M, Philippe J, Silvestre JS, Van Rooijen N, Corvol P, Nicoletti A, Chazaud B, Germain S. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS One. 2008; 3:e3950. PMID: 19079608.

43. Kodelja V, Müller C, Tenorio S, Schebesch C, Orfanos CE, Goerdt S. Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology. 1997; 197:478–493. PMID: 9413747.

SUPPLEMENTARY MATERIALS
Supplementary data including one figure and two tables can be found with this article online at http://pdf.medrang.co.kr/paper/pdf/Kjpp/Kjpp020-06-12-s001.pdf.
Supplementary Fig. 1
Typical MSCs characterization. To characterize mesenchymal stem cells extracted from human bone marrow, (A) we used MSCs specific surface markers for FACS analysis. FACS revealed positivity for CD73 and CD105 and negativity for CD34 and CD45. The gray color indicates the negative control. (B) To check differentiation capacity into adipocyte or osteocyte, differentiated adipocyte and osteocyte were stained with oil red O or alkaline phosphatase, respectively.
Supplementary Table 1
Antibodies list used for immunofluorescence, flow cytometry, ELISA and immunoblotting
Fig. 1
Increased MSCs survival after double injections of MSCs in the limb ischemia animal model.
(A) Double stained confocal microscopic images showing that distribution of CD44 positive MSCs (green) in ischemic muscle at 4 weeks after first injections depended on injection frequency and MSCs dose. Below graph came from representative pictures (above) and arrows indicated existed MSCs in the ischemic muscles. (B) CD29 and CD44 mRNA levels were assessed by qRT-PCR in the ischemic muscles of mice treated single or double with high or low dose of MSCs injection. Scale bar=100 µm, original magnification=200×, None; no signal detectable, *Different (p<0.05) versus a single injection of low dose, §Different (p<0.05) versus double low dose of MSCs injections, †Different (p<0.05) versus double high dose of MSCs injections.
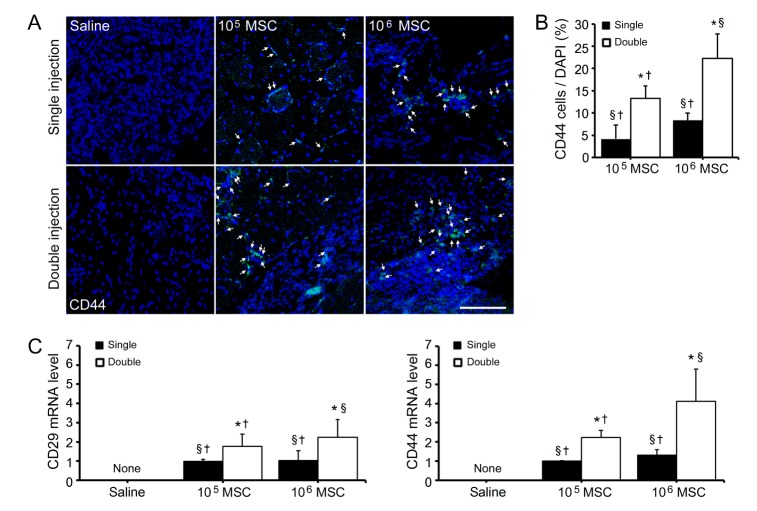
Fig. 2
Paracrine induction of angiogenesis by MSCs secretions.
HUVECs were treated with MSCs conditioned medium (MSC-CM) of PBS (control) to determine the ability of MSCs of promote angiogenesis, (A) HUVECs migration, (B) tube-like structure formation, and (C) HUVECs proliferation. Scale bar=1000 pixels, original magnification=40× *p<0.05 vs. PBS treated HUVECs (D) Levels of ANG-1, HGF, and VEGF in MSC-CM were measured by ELISA. The ELISA plot shows fold levels of proteins in MSC-CM versus basal medium (Basal-M). (E) Immunoblotting results showing that the expressions of pAKT and MAPK pathway-related molecules in Basal-M and MSC-CM treated HUVECs in vitro. *p<0.05 vs. Basal-M treated HUVECs (F) Confocal microscopic images showing the effects of MSCs dose and injection frequency on vWF (green) positive blood vessels and nuclei (DAPI; blue) in ischemic muscle. Vessel sizes were calculated by Zen 2009 software and described in graph. Scale bar=100 µm, original magnification=200 ×, *Different (p<0.05) versus a single injection of low dose, §Different (p<0.05) versus double low dose of MSCs injections, †Different (p<0.05) versus double high dose of MSCs injection.
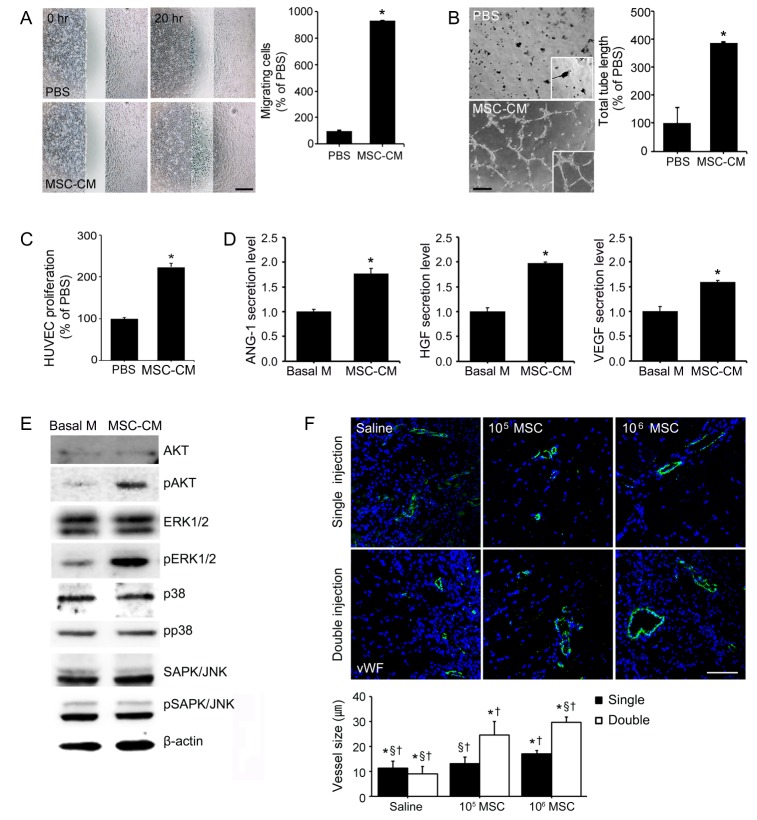
Fig. 3
High dose of MSCs injection enhanced M2 macrophage polarization in the limb ischemia animal model.
(A) Triple stained microscopic images showing total activated macrophages (Iba1), M2 macrophage marker (Arg 1) and nuclei (DAPI; blue). (B) Graphs were derived from representative microscopic images. The graph in the left panel shows numbers of activated macrophages (Iba1) and the right panel shows the ratio of Arg1 positive cells to Iba1 cells. Scale bar=100 µm, original magnification=200×, *Different (p<0.05) versus a single injection of low dose, §Different (p<0.05) versus double low dose of MSCs injections, †Different (p<0.05) versus double high dose of MSCs injection.
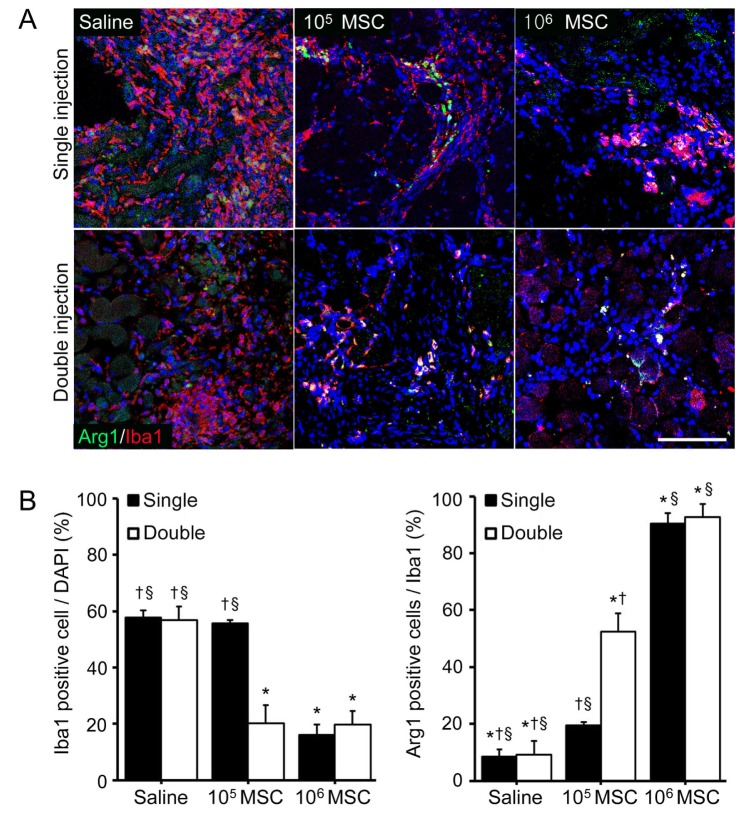
Fig. 4
Protective effects of double injections of MSCs in the limb ischemia animal model.
(A) Fibrotic fibers (blue) in ischemic muscle were confirmed by Masson's trichrome staining and (B) apoptotic cells (arrows) were TUNEL stained. Scale bar=100 µm (C) Skin color immediately changed after ferric chloride had been applied to femoral arteries followed by MSCs injection visibly ameliorated limb ischemia. Limb salvage, necrosis and amputation ratios were described by white, gray and black color, respectively. *Different (p<0.05) versus a single injection of low dose, §Different (p<0.05) versus double low dose of MSCs injections, †Different (p<0.05) versus double high dose of MSCs injection.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download