INTRODUCTION
METHODS
Cell culture
Cell viability assay
Reverse transcriptase-polymerase chain reaction (RT-PCR) and real-time quantitative RT-PCR (qRT-PCR)
Western blot analysis
Osteogenic induction
Chondrogenic induction
Adipogenic induction
Statistical analysis
RESULTS
Time- and dose-dependent viability kinetics of CoCl2 treated C3H/10T1/2 cells
 | Fig. 1Effects of CoCl2 on cell viability of C3H/10T1/2 cells.C3H/10T1/2 cells were cultured in the 2% serum medium in the presence of different CoCl2 concentrations for 48 h (A) and in the presence of 0.1 or 0.25 mM CoCl2 for a designated time (B). Cell viability was determined by the MTT assay. Each value is the mean±SD of triplicate independent experiments. *p<0.05, as compared to control.
|
CoCl2 increases expressions of HIF-1α and VEGF
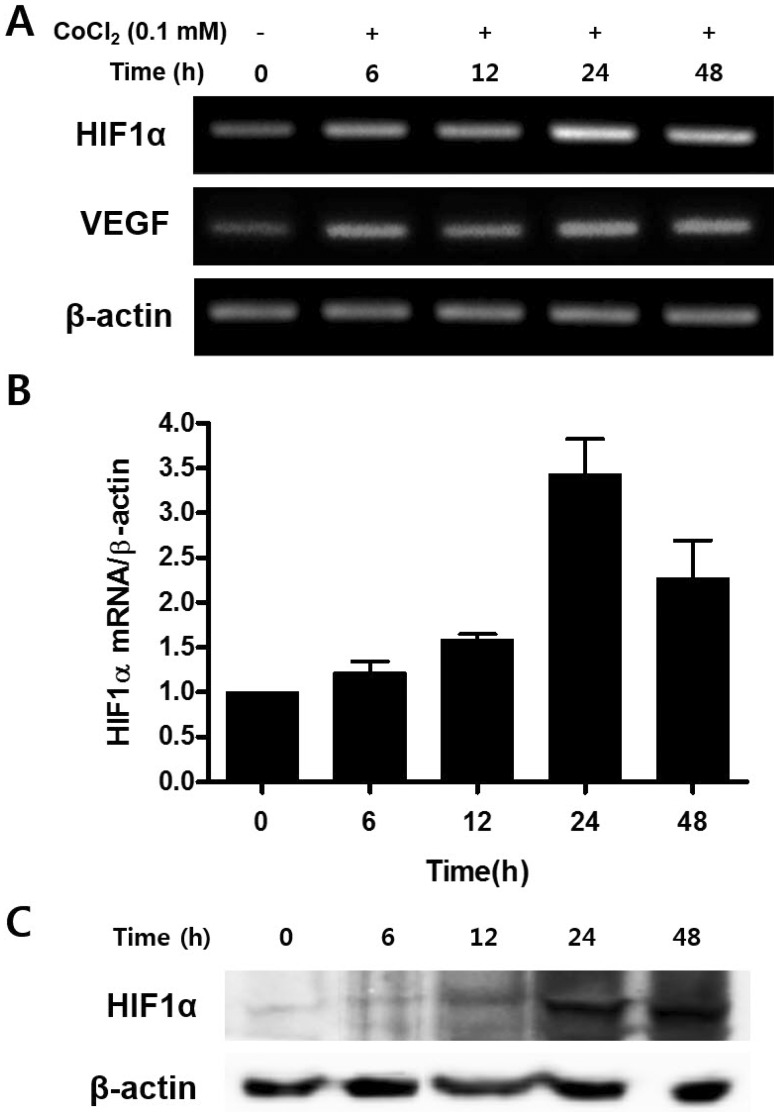 | Fig. 2Effect of CoCl2 in the expression of HIF-1α and VEGF mRNA.C3H/10T1/2 cells were seeded in 60-mm culture dishes at a density of 1×105 cells and incubated in the growth medium containing 0.1 mM CoCl2 for 48 h. At the indicated times, total RNA and cell lysates were isolated and RT-PCR analysis (A), quantitative real time RT-PCR analysis (B), and Western blotting analysis (C) was done.
|
CoCl2 enhances osteogenic differentiation of C3H/10T1/2 cells
 | Fig. 3Effects of treatment of CoCl2 on osteogenic differentiation.(A) C3H/10T1/2 cells were pre-incubated with 0.1 mM CoCl2 for 0 h, 24 h or 48 h. After incubation, the cells were replaced with osteogenic medium and cultured for 18 days, prior to staining with Alizarin red S. (B) Results from (A) were quantified by spectrophotometry. (C) C3H/10T1/2 cells were preincubated with 0.1 mM CoCl2 for 0 h, 24 h, or 48 h. After incubation, cells were cultured with osteogenic medium for 3 days. Total cellular RNA was extracted, and gene expression of osteogenic markers was detected by semi-quantitative RT-PCR. Expression of actin was examined in the same sample as a control for the amount of present reverse-transcribed cDNA. (D) Effects of treatment of CoCl2 during osteoblast differentiation. C3H/10T1/2 cells were pre-incubated with 0.1 mM CoCl2 for 0 h, 24 h, or 48 h. After incubation, the cells were cells were replaced with an osteogenic medium and cultured for 10 days. At indicated times, total cellular RNA was extracted and gene expression of osteogenic markers was assessed by qRT-PCR. Values shown are normalized to β-actin levels. The data represent the mean±S.D. from triplicate independent experiments (*p<0.05).
|
CoCl2 enhances chondrogenic differentiation of C3H/10T1/2 cells
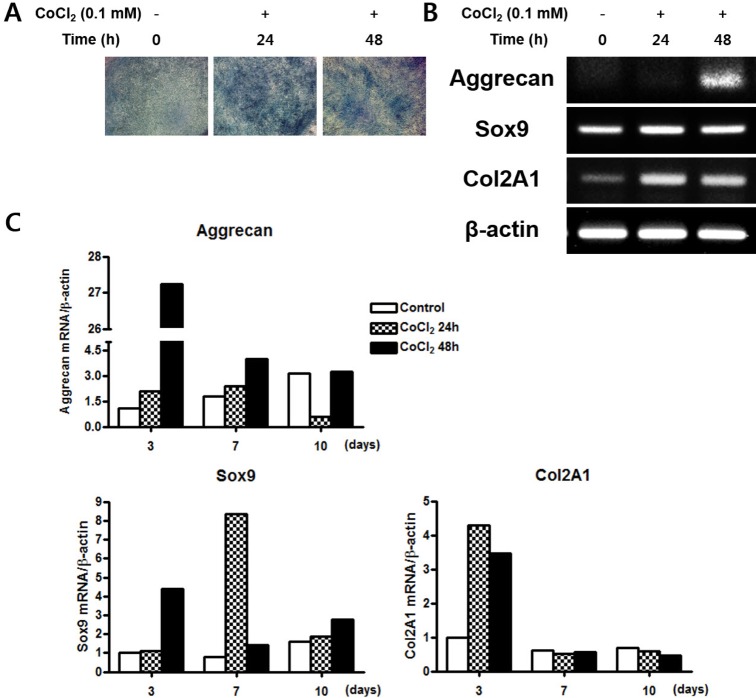 | Fig. 4Effects of treatment of CoCl2 on the chondrogenic differentiation.(A) C3H/10T1/2 cells were pre-incubated with 0.1 mM CoCl2 for 0 h, 24 h, or 48 h. After incubation, the cells shifted to a chondrogenic medium and cultured for 14 days. The cells were then stained with Alcian blue. (B) C3H/10T1/2 cells were pre-incubated with 0.1 mM CoCl2 for 0 h, 24 h, or 48 h. After incubation, the cells were shifted to a chondrogenic medium and cultured for 3 days. Total cellular RNA was extracted and gene expression of the chondrogenic markers aggrecan, sox9, and Col 2A1 was assessed by semi- quantitative RT-PCR. Expression of actin was examined in the same sample as a control for the amount of present reverse-transcribed cDNA. (C) Effects of treatment of CoCl2 during chondrocyte differentiation. C3H/ 10T1/2 cells were pre-incubated with 0.1 mM CoCl2 for 0 h, 24 h, or 48 h. After incubation, the cells were replaced with chondrogenic medium and cultured for 10 days. At indicated times, total cellular RNA was extracted and gene expression of the chondrogenic makers aggrecan, sox9, and Col 2A1 was assessed by qRT-PCR. Values shown are normalized to β-actin levels.
|
CoCl2 suppresses adipogenic differentiation of C3H/10T1/2 cells
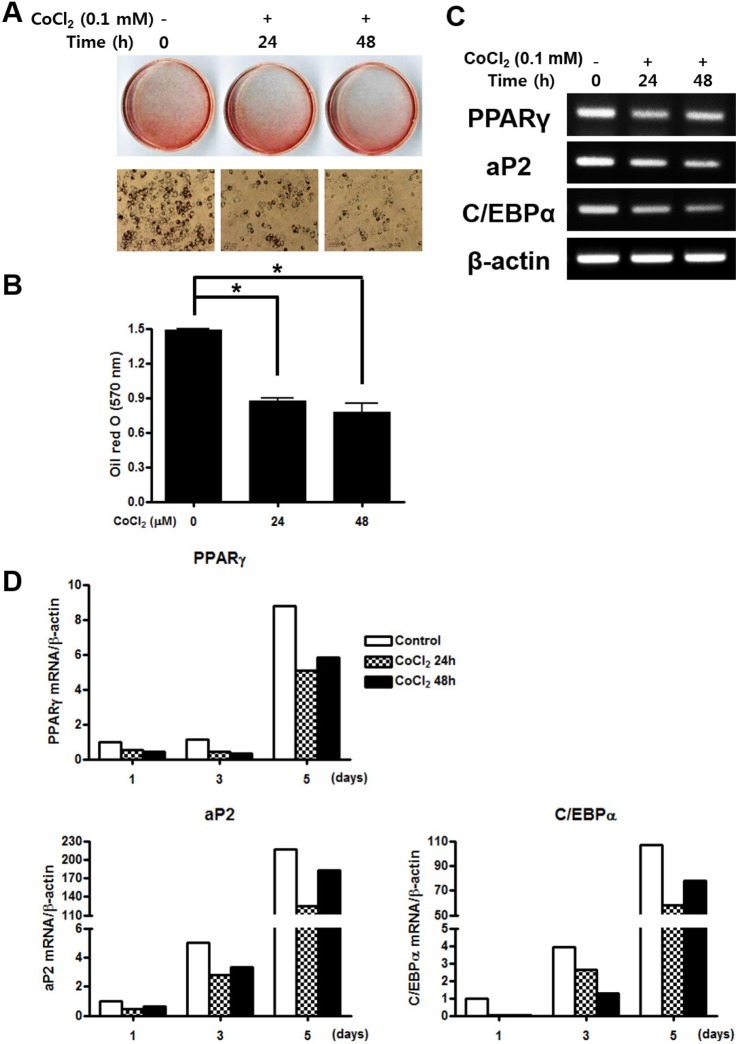 | Fig. 5Effects of treatment of CoCl2 on the adipogenic differentiation.(A) C3H/10T1/2 cells were pre-incubated with 0.1 mM CoCl2 for 0 h, 24 h, or 48 h. After incubation, the cells were cultured with adipogenic medium for 5 days then stained with Oil red s. (B) The degree of lipid accumulation as quantified by spectrophotometry. (C) Total cellular RNA was extracted and gene expression of the adipogenic markers PPARγ, aP2, and C/EBPα was assessed by semi-quantitative RT-PCR. Expression of actin was examined in the same sample as a control for the amount of present reverse-transcribed cDNA. (D) Effects of treatment of CoCl2 during adipocyte differentiation. C3H/10T1/2 cells were preincubated with 0.1 mM CoCl2 for 0 h, 24 h, or 48 h. After incubation, the cells were cells were replaced with adipogenic medium and cultured for 10 days. At indicated times, total cellular RNA was extracted and gene expression of the adipogenic makers PPARγ, aP2, and C/EBPα was analyzed by qRT-PCR. Values shown are normalized to β-actin levels. The data represent the mean±S.D. from triplicate independent experiments (*p<0.05).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download