1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.
2. Kim JH, Kim H, Lee KY, Kang JW, Lee KH, Park SY, Yoon HI, Jheon SH, Sung SW, Hong YC. Aryl hydrocarbon receptor gene polymorphisms affect lung cancer risk. Lung Cancer. 2007; 56:9–15.
3. Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact. 2000; 129:77–97.
4. Cadenas E. Antioxidant and prooxidant functions of DT-diaphorase in quinone metabolism. Biochem Pharmacol. 1995; 49:127–140.
5. O'Brien PJ. Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact. 1991; 80:1–41.
6. Asher G, Lotem J, Cohen B, Sachs L, Shaul Y. Regulation of p53 stability and p53-dependent apoptosis by NADH quinone oxidoreductase 1. Proc Natl Acad Sci U S A. 2001; 98:1188–1193.
7. Lind C, Cadenas E, Hochstein P, Ernster L. DT-diaphorase: purification, properties, and function. Methods Enzymol. 1990; 186:287–301.
8. Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol. 2004; 65:1238–1247.
9. Wattenberg LW. Chemoprevention of cancer. Cancer Res. 1985; 45:1–8.
10. Chao C, Zhang ZF, Berthiller J, Boffetta P, Hashibe M. NAD(P)H:quinone oxidoreductase 1 (NQO1) Pro187Ser polymorphism and the risk of lung, bladder, and colorectal cancers: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006; 15:979–987.
11. Chen H, Lum A, Seifried A, Wilkens LR, Le Marchand L. Association of the NAD(P)H:quinone oxidoreductase 609C→T polymorphism with a decreased lung cancer risk. Cancer Res. 1999; 59:3045–3048.
12. Hamajima N, Matsuo K, Iwata H, Shinoda M, Yamamura Y, Kato T, Hatooka S, Mitsudomi T, Suyama M, Kagami Y, et al. NAD(P)H: quinone oxidoreductase 1 (NQO1) C609T polymorphism and the risk of eight cancers for Japanese. Int J Clin Oncol. 2002; 7:103–108.
13. Lawson KA, Woodson K, Virtamo J, Albanes D. Association of the NAD(P)H: quinone oxidoreductase (NQO1) 609C->T polymorphism with lung cancer risk among male smokers. Cancer Epidemiol Biomarkers Prev. 2005; 14:2275–2276.
14. Lewis SJ, Cherry NM, Niven RM, Barber PV, Povey AC. Polymorphisms in the NAD(P)H: quinone oxidoreductase gene and small cell lung cancer risk in a UK population. Lung Cancer. 2001; 34:177–183.
15. Lin P, Hsueh YM, Ko JL, Liang YF, Tsai KJ, Chen CY. Analysis of NQO1, GSTP1, and MnSOD genetic polymorphisms on lung cancer risk in Taiwan. Lung Cancer. 2003; 40:123–129.
16. Menzel HJ, Sarmanova J, Soucek P, Berberich R, Grünewald K, Haun M, Kraft HG. Association of NQO1 polymorphism with spontaneous breast cancer in two independent populations. Br J Cancer. 2004; 90:1989–1994.
17. Anwar A, Siegel D, Kepa JK, Ross D. Interaction of the molecular chaperone Hsp70 with human NAD(P)H:quinone oxidoreductase 1. J Biol Chem. 2002; 277:14060–14067.
18. Siegel D, Anwar A, Winski SL, Kepa JK, Zolman KL, Ross D. Rapid polyubiquitination and proteasomal degradation of a mutant form of NAD(P)H: quinone oxidoreductase 1. Mol Pharmacol. 2001; 59:263–268.
19. Ross D, Traver RD, Siegel D, Kuehl BL, Misra V, Rauth AM. A polymorphism in NAD(P)H:quinone oxidoreductase (NQO1): relationship of a homozygous mutation at position 609 of the NQO1 cDNA to NQO1 activity. Br J Cancer. 1996; 74:995–996.
20. Siegel D, McGuinness SM, Winski SL, Ross D. Genotype-phenotype relationships in studies of a polymorphism in NAD(P)H:quinone oxidoreductase 1. Pharmacogenetics. 1999; 9:113–121.
21. Traver RD, Horikoshi T, Danenberg KD, Stadlbauer TH, Danenberg PV, Ross D, Gibson NW. NAD(P)H:quinone oxidoreductase gene expression in human colon carcinoma cells: characterization of a mutation which modulates DT-diaphorase activity and mitomycin sensitivity. Cancer Res. 1992; 52:797–802.
22. Traver RD, Siegel D, Beall HD, Phillips RM, Gibson NW, Franklin WA, Ross D. Characterization of a polymorphism in NAD(P)H: quinone oxidoreductase (DT-diaphorase). Br J Cancer. 1997; 75:69–75.
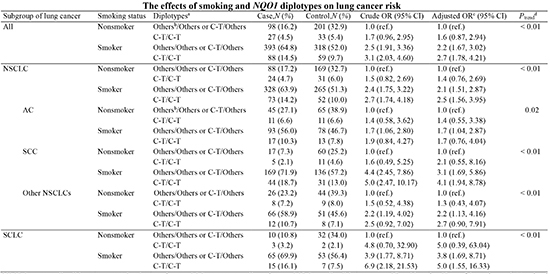
23. Eom SY, Zhang YW, Kim SH, Choe KH, Lee KY, Park JD, Hong YC, Kim YD, Kang JW, Kim H. Influence of NQO1, ALDH2, and CYP2E1 genetic polymorphisms, smoking, and alcohol drinking on the risk of lung cancer in Koreans. Cancer Causes Control. 2009; 20:137–145.
24. Guo S, Gao M, Li X, Li Y, Chu S, Zhu D, Niu W. Lack of association between NADPH quinone oxidoreductase 1 (NQO1) gene C609T polymorphism and lung cancer: a case-control study and a meta-analysis. PLoS One. 2012; 7:e47939.
25. Liu Y, Zhang D. The NQO1 C609T polymorphism and risk of lung cancer: a meta-analysis. Asian Pac J Cancer Prev. 2011; 12:3091–3095.
26. Vineis P, Veglia F, Garte S, Malaveille C, Matullo G, Dunning A, Peluso M, Airoldi L, Overvad K, Raaschou-Nielsen O, et al. Genetic susceptibility according to three metabolic pathways in cancers of the lung and bladder and in myeloid leukemias in nonsmokers. Ann Oncol. 2007; 18:1230–1242.
27. Yang M, Choi Y, Hwangbo B, Lee JS. Combined effects of genetic polymorphisms in six selected genes on lung cancer susceptibility. Lung Cancer. 2007; 57:135–142.
28. Kim JH, Hong YC. No Association between Tumor Necrosis Factor-alpha Gene Polymorphisms and Lung Cancer Risk. Environ Health Toxicol. 2013; 28:e2013012.
29. Kim JH, Kim H, Lee KY, Choe KH, Ryu JS, Yoon HI, Sung SW, Yoo KY, Hong YC. Genetic polymorphisms of ataxia telangiectasia mutated affect lung cancer risk. Hum Mol Genet. 2006; 15:1181–1186.
30. Lee KM, Choi JY, Park SK, Chung HW, Ahn B, Yoo KY, Han W, Noh DY, Ahn SH, Kim H, et al. Genetic polymorphisms of ataxia telangiectasia mutated and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005; 14:821–825.
31. Cauffiez C, Lo-Guidice JM, Quaranta S, Allorge D, Chevalier D, Cenée S, Hamdan R, Lhermitte M, Lafitte JJ, Libersa C, et al. Genetic polymorphism of the human cytochrome CYP2A13 in a French population: implication in lung cancer susceptibility. Biochem Biophys Res Commun. 2004; 317:662–669.
32. Dally H, Edler L, Jäger B, Schmezer P, Spiegelhalder B, Dienemann H, Drings P, Schulz V, Kayser K, Bartsch H, et al. The CYP3A4*1B allele increases risk for small cell lung cancer: effect of gender and smoking dose. Pharmacogenetics. 2003; 13:607–618.
33. Simonato L, Agudo A, Ahrens W, Benhamou E, Benhamou S, Boffetta P, Brennan P, Darby SC, Forastiere F, Fortes C, et al. Lung cancer and cigarette smoking in Europe: an update of risk estimates and an assessment of inter-country heterogeneity. Int J Cancer. 2001; 91:876–887.
34. Wang H, Tan W, Hao B, Miao X, Zhou G, He F, Lin D. Substantial reduction in risk of lung adenocarcinoma associated with genetic polymorphism in CYP2A13, the most active cytochrome P450 for the metabolic activation of tobacco-specific carcinogen NNK. Cancer Res. 2003; 63:8057–8061.
35. Yang P, Wentzlaff KA, Katzmann JA, Marks RS, Allen MS, Lesnick TG, Lindor NM, Myers JL, Wiegert E, Midthun DE, et al. Alpha1-antitrypsin deficiency allele carriers among lung cancer patients. Cancer Epidemiol Biomarkers Prev. 1999; 8:461–465.
36. Kristiansen OP, Larsen ZM, Johannesen J, Nerup J, Mandrup-Poulsen T, Pociot F. Danish IDDM Epidemiology and Genetics Group and The Danish Study Group of Diabetes in Childhood. No linkage of P187S polymorphism in NAD(P)H: quinone oxidoreductase (NQO1/DIA4) and type 1 diabetes in the Danish population. DIEGG and DSGD. Hum Mutat. 1999; 14:67–70.
37. Wang G, Zhang L, Li Q. Genetic polymorphisms of GSTT1, GSTM1, and NQO1 genes and diabetes mellitus risk in Chinese population. Biochem Biophys Res Commun. 2006; 341:310–313.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download