Abstract
Figures and Tables
 | Fig. 3Forest plots. The effects of MSC treatment on total bilirubin (A) and serum albumin (B) were estimated after 6 months of treatment. SD, standard deviation; N, number of patients; CI, confidence interval; E, experimental; C, control; IV, inverse variance. |
Table 1
Summary of the included studies
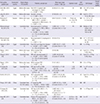
| First author, publish year | Conducted country | Study design, F/U (month) | Patients, sample size | Mean age range (mean ± SD), (yr) | Injection route | Cell source | Cell dosage | Overall study quality |
|---|---|---|---|---|---|---|---|---|
| Amin MA, 2013 (11) | Egypt |
Before-after study 6 |
Post-HCV LC (patient with end-stage LC), Child C | 42-60 (51.3 ± 6.2) | IS | BM | 10 × 106 in 10 mL PBS | - |
| n = 20 (M:F = 14:6) | ||||||||
| Jang YO, 2014 (12) | Korea |
Before-after study 6 |
Alcoholic cirrhosis | 37-60 (50 ± 8) | HA | BM | Each 5 × 107 in 10 mL NS, twice | - |
| n = 11 (M:F = 10:1) | ||||||||
| Kharaziha P, 2009 (13) | Sweden |
Before-after study 6 |
LC, end stage liver disease (HBV 4, HCV 1, alcoholic 1, cryptogenic 2), MELD score ≥ 10 | 38-67 (55.63 ± 10.45) | Portal vein (n = 6) or PV (n = 2) | BM | 3× 107-5 × 107 in 10 mL NS | - |
| n = 8 (M:F = 4:4) | ||||||||
| Mohamadnejad M, 2013 (14) | Iran |
Controlled trials 12 |
Decompensated LC (cirrhosis cryptogenic 11, PBC 2, HBV 2, HCV 1, AIH 9) |
1) MSC 43.1 ± 17.6 2) Control 34.6 ± 13.8 |
PV | BM | Median of 195 million (120-295 million) in 100 mL NS | + |
| n = 25 (M:F = 13:12), 1) MSC (n = 14), 2) Control (placebo) (n = 11) | ||||||||
| Mohamadnejad M, 2007 (15) | Iran |
Case series 12 |
Decompensated LC (cryptogenic 3, AIH1) | 34-56 (47.3) | PV | BM | 31.7 × 106 (10.2-60 × 106) in 20 mL NS | - |
| n = 4 (M:F = 1:3) | ||||||||
| Salama H, 2014 (16) | Egypt |
Controlled trials 6 |
Post-HCV end-stage liver disease |
1) MSC 50.27 ± 6.05 2) Control 50.9 ± 7.23 |
PV | BM | 1× 106/kg | ++ |
| n = 40 (M:F = 33:7) 1) MSC (n = 20), 2) Control (n = 20) | ||||||||
| Wang L, 2013 (17) | China |
Before-after study 12 |
UDCA-resistant PBC | 33-58 (49) | PV | UC | Each 0.5 × 106/kg in NS, thrice | - |
| n = 7 (M:F = 1:6) | ||||||||
| Zhang Z, 2012 (18) | China |
Controlled trials 12 |
HBV with decompensated LC |
1) MSC 48 (25-64) 2) Control 47 (29-64) |
PV | UC | Each 0.5 × 106/kg in NS, thrice | + |
| n = 45 (M:F = 40:5) 1) MSC (n = 30), 2) Control (saline) (n = 15) | ||||||||
| Wang L, 2014 (19) | China |
Before-after study 12 |
UDCA-resistant PBC | 31-61 (49.1) | PV | BM | 3-5 × 105/kg | - |
| n = 10 (M:F = 1:9) | ||||||||
| El-Ansary M, 2010 (20) | Egypt |
Controlled trials 6 |
CHF due to HCV or HBV, MELD > 12, Child C , LC |
1) IS 48.50 ± 11.09 (32-69) 2) PV 50.83 ± 6.88 (43-59) |
1) IS 2) PV |
BM | 1× 107 in 5 mL NS | + |
| n = 12 (M:F = 8:4), 1) IS (n = 6), 2) PV (n = 6) | ||||||||
| Shi M, 2012 (21) | China |
Controlled trials 18 |
ACLF associated HBV |
1) MSC m 40 (24-59) 2) Control m 45 (26-62) |
PV | UC | Each 0.5 × 106/kg, thrice | + |
| n = 43 (M:F = 35:8), 1) MSC (n = 24), 2) Control (saline) (n = 19) | ||||||||
| Peng L, 2011 (22) | China |
Controlled trials 45 (192 weeks) |
LF caused by HBV |
1) MSC 42.19 ± 10.80 2) Control 42.22 ± 11.47 |
HA | BM | NR | + |
| n = 158 (M:F = 149:9), 1) MSC (n = 53), 2) Control (n = 105) | ||||||||
| El-Ansary M, 2012 (23) | Egypt |
Controlled trials 6 |
CHF due to HCV, MELD > 12, Child C, LC |
1) MSC 48.0 ± 7.4 (32.0-60.0) 2) Control 51.6 ± 7.2 (39.0-60.0) |
PV | BM | 1 × 106/kg in NS | + |
| n = 25 (M:F = 19:6), 1) MSC (n = 15), 2) Control (n = 10) | ||||||||
| Amer ME, 2011 (24) | Egypt |
Controlled trials 6 |
Chronic HCV-associated LF, MELD > 25, Child C, LC |
1) MSC 50.5 + 4.1 2) Control 45-55 + 3.6 |
(1) IS (n = 10) (2) IH (n = 10) |
BM | 2× 107 | ++ |
| n = 40 (M:F = 33:7) 1) MSC (n = 20), 2) Control (n = 20) |
AIH, auto immune hepatitis; BM, bone marrow; CHF, chronic hepatic failure; Child C, end-stage liver cirrhosis; HA, hepatic artery; HBV, hepatitis B virus; HCV, hepatitis C virus; IH, intrahepatic; IS, intrasplenic; LC, liver cirrhosis; LF, liver failure; MELD, model for end-stage liver disease; MSC, mesenchymal stem cell; NS, normal saline; PBC, primary biliary cirrhosis; PBS, phosphate-buffered saline; PV, peripheral vein; RCT, randomized controlled trials; UC, umbilical cord; UDCA, ursodeoxycholic acid; ACLF, acute-on-chronic liver failure; NR, not report.
Table 2
Experimental group vs. control group change value

| Group | No. of patients | Baseline | 3 months | 6 months | 9 months | 12 months | Unit | ||
|---|---|---|---|---|---|---|---|---|---|
| INR | Mohamadnejad M, 2013 (14) | Experimental | 14 | 1.5 ± 0.5 | 1.8 ± 0.5 | 1.5 ± 0.4 | NR | 1.5 ± 0.3 | |
| Control | 11 | 1.6 ± 0.2 | 1.6 ± 0.4 | 1.4 ± 0.3 | NR | 1.3 ± 0.4 | |||
| Salama H, 2014 (16) | Experimental | 20 | 1.53 ± 0.19 | 1.47 ± 0.23 | 1.52 ± 0.36 | E | E | ||
| Control | 20 | 1.66 ± 0.33 | 0.73 ± 0.4 | 1.84 ± 0.39 | E | E | |||
| Zhang Z, 2012 (18) | Experimental | 30 | 1.4 ± 0.3 | 1.3 ± 0.15 | 1.3 ± 0.1 | 1.28 ± 0.1 | 1.25 ± 0.12 | ||
| Control | 15 | 1.3 ± 0.15 | 1.2 ± 0.1 | 1.2 ± 0.12 | 1.25 ± 0.15 | 1.2 ± 0.12 | |||
| PT | Salama H, 2014 (16) | Experimental | 20 | 55.3 ± 9.06 | 59.45 ± 15.23 | 57.59 ± 14.68 | E | E | Prothrombin con centration % |
| Control | 20 | 52.85 ± 10.16 | 50.45 ± 11.42 | 45.03 ± 10.92 | E | E | |||
| Zhang Z, 2012 (18) | Experimental | 30 | 58 ± 14 | 66 ± 12 | 72 ± 20 | 70 ± 12 | 72 ± 14 | Prothrombin activity % | |
| Control | 15 | 64 ± 12 | 75 ± 15 | 74 ± 14 | 70 ± 14 | 72 ± 10 | |||
| Shi M, 2012 (21) | Experimental | 24 | 35 ± 4 | 72 ± 20 | 76 ± 17 | 82 ± 16 | 85 ± 14 | Prothrombin activity % | |
| Control | 19 | 32 ± 9 | 58 ± 6 | 64 ± 11 | 66 ± 14 | 67 ± 8 | |||
| Peng L, 2011 (22) | Experimental | 53 | 26.25 ± 5.34 | 14.82 ± 2.53 | 16.23 ± 2.56 | 15.64 ± 3.17 | 16.32 ± 2.97 | Prothrombin time (sec) | |
| Control | 105 | 25.95 ± 5.72 | 19.25 ± 3.66 | 17.53 ± 3.31 | 17.19 ± 3.07 | 17.75 ± 3.14 | |||
| El-Ansary, 2012 (23) | Experimental | 15 | 44.3 ± 14.7 | 51.6 ± 13.6 | 50 ± 15 | E | E | Prothrombin concentration % | |
| Control | 10 | 41.7 ± 14.2 | 39.5 ± 15.5 | 36.8 ± 16 | E | E | |||
| S.Alb | Mohamadnejad M, 2013 (14) | Experimental | 14 | 3.3 ± 0.6 | 3.3 ± 0.7 | 3.3 ± 0.5 | NR | 3.1 ± 0.8 | g/dL |
| Control | 11 | 3.5 ± 0.6 | 3.8 ± 0.5 | 3.9 ± 0.7 | NR | 3.9 ± 0.3 | |||
| Salama H, 2014 (16) | Experimental | 20 | 2.59 ± 0.28 | 2.99 ± 0.26 | 3.06 ± 0.36 | E | E | g/dL | |
| Control | 20 | 2.62 ± 0.37 | 2.63 ± 0.3 | 2.43 ± 0.36 | E | E | |||
| Zhang Z, 2012 (18) | Experimental | 30 | 28 ± 7 | 32.5 ± 5.5 | 33 ± 4 | 33 ± 7 | 35 ± 5 | g/L | |
| Control | 15 | 28 ± 18 | 30 ± 5 | 32 ± 7 | 30 ± 5 | 30 ± 3 | |||
| Shi M, 2012 (21) | Experimental | 24 | 3.14 ± 0.27 | 3.47 ± 0.7 | 3.82 ± 0.59 | NR | 4.18 ± 0.53 | g/dL | |
| Control | 19 | 2.82 ± 0.39 | 2.83 ± 0.05 | 3.26 ± 0.13 | NR | 3.08 ± 0.4 | |||
| Peng L, 2011 (22) | Experimental | 53 | 29.67 ± 3.14 | 36.75 ± 2.27 | 36.93 ± 2.43 | 37.50 ± 2.31 | 36.83 ± 2.18 | g/L | |
| Control | 105 | 29.40 ± 3.92 | 33.93 ± 1.98 | 34.33 ± 2.61 | 36.17 ± 1.97 | 36.73 ± 2.71 | |||
| T.Bil | Mohamadnejad M, 2013 (14) | Experimental | 14 | 2.6 ± 1.4 | 4.1 ± 2.4 | 2.2 ± 1 | NR | 2.2 ± 1.4 | mg/dL |
| Control | 11 | 3.5 ± 2.2 | 5.3 ± 1.9 | 3 ± 1.6 | NR | 2.7 ± 1.4 | |||
| Salama H, 2014 (16) | Experimental | 20 | 1.88 ± 1.05 | 1.82 ± 1.3 | 2.06 ± 1.26 | E | E | mg/dL | |
| Control | 20 | 2.51 ± 0.94 | 4.02 ± 3.29 | 4.24 ± 2.48 | E | E | |||
| Zhang Z, 2012 (18) | Experimental | 30 | 42.0 ± 22.0 | 30.0 ± 17.0 | 29.0 ± 16.0 | 28.0 ± 18.0 | 26.0 ± 18.0 | µM | |
| Control | 15 | 48.0 ± 7.0 | 38.0 ± 12.0 | 36.0 ± 14.0 | 30.0 ± 9.0 | 29.0 ± 6.0 | |||
| Shi M, 2012 (21) | Experimental | 24 | 325.0 ± 124.0 | 50.0 ± 50.0 | 45.0 ± 40.0 | 28.0 ± 10.0 | 25.0 ± 10.0 | µM | |
| Control | 19 | 330.0 ± 130.0 | 75.0 ± 20.0 | 65.0 ± 40.0 | 50.0 ± 35.0 | 52.0 ± 60.0 | |||
| Peng L, 2011 (22) | Experimental | 53 | 201.170 ± 75.450 | 27.080 ± 6.390 | 72 ± 20 | 70 ± 12 | 72 ± 14 | µM | |
| Control | 105 | 295.730 ± 56.020 | 42.530 ± 21.170 | 22.170 ± 4.620 | 27.600 ± 10.290 | 26.830 ± 5.780 | |||
| MELD | Mohamadnejad M, 2013 (14) | Experimental | 14 | 15.4 ± 5.4 | 15.3 ± 8.2 | NR | NR | 14 ± 3.6 | |
| Control | 11 | 14.4 ± 3.7 | 14.7 ± 5.1 | NR | NR | 12.5 ± 4.3 | |||
| Shi M, 2012 (21) | Experimental | 24 | 24.05 ± 4.0 | 9.2 ± 5.8 | NR | NR | NR | ||
| Control | 19 | 26.5 ± 4.6 | 14.7 ± 4.5 | NR | NR | NR | |||
| Peng L, 2011 (22) | Experimental | 53 | 29.58 ± 0.93 | 15.29 ± 2.25 | 14.67 ± 2.89 | 15.55 ± 1.73 | 17.39 ± 2.68 | ||
| Control | 105 | 29.62 ± 3.75 | 19.73 ± 7.49 | 18.37 ± 2.91 | 18.79 ± 2.73 | 18.0 ± 2.52 | |||
| Amer ME, 2011 (24) | Experimental | 20 | 11.57 ± 2.26 | NR | 11.66 ± 2.29 | E | E | ||
| Control | 20 | 12.55 ± 2.61 | NR | 14.11 ± 2.73 | E | E | |||
| Child | Mohamadnejad M, 2013 (14) | Experimental | 14 | 7.7 ± 2.5 | 7 ± 2.9 | NR | NR | 7.2 ± 1.7 | |
| Control | 11 | 8.3 ± 1.8 | 6.8 ± 2.1 | NR | NR | 6.6 ± 1.5 | |||
| Amer ME, 2011 (24) | Experimental | 20 | 11.45 ± 1.09 | NR | 11.45 ± 0.95 | E | E | ||
| Control | 20 | 11.7 ± 1.08 | NR | 12.35 ± 0.67 | E | E |
Table 3
Pre-post change value

| Study | Baseline | 3 months | 6 months | 9 months | 12 months | Significant improved | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M pre | SD pre | No. | M post | SD post | M post | SD post | M post | SD post | M post | SD post | ||||
| INR | Amin MA, 2013 (11) | 2.5 | 0.5 | 20 | 1.7 | 0.2 | 1.2 | 0.1 | E | E | E | E | Y | |
| Jang YO, 2014 (12) | 1.2 | 0.1 | 11 | NR | NR | 1.1 | 0.1 | E | E | E | E | Y | ||
| Kharaziha P, 2009 (13) | 1.9 | 0.4 | 8 | 1.4 | 0.5 | NR | NR | E | E | E | E | Y | ||
| Mohamadnejad M, 2013 (14) | 1.5 | 0.5 | 14 | 1.8 | 0.5 | NR | NR | NR | NR | 1.5 | 0.3 | N | ||
| Mohamadnejad M, 2007 (15) | 1.88 | 0.34 | 4 | NR | NR | 1.4 | 0.41 | NR | NR | 1.53 | 0.15 | Y | ||
| Salama H, 2014 (16) | 1.53 | 0.19 | 20 | 1.47 | 0.23 | 1.52 | 0.36 | E | E | E | E | N | ||
| Wang L, 2013 (17) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | N | ||
| Zhang Z, 2012 (18) | 1.4 | 0.3 | 30 | 1.3 | 0.15 | 1.3 | 0.1 | 1.28 | 0.1 | 1.25 | 0.12 | Y | ||
| Amer ME, 2011 (24) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | N | ||
| PT | Amin MA, 2013 (11) | 22.5 | 2.5 | 20 | 19 | 1.5 | 17 | 1 | E | E | E | E | Y | |
| Mohamadnejad M, 2013 (14) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | N | ||
| Mohamadnejad M, 2007 (15) | 18.35 | 1.89 | 4 | NR | NR | 15.18 | 2.57 | NR | NR | 16.05 | 1.31 | Y | ||
| Salama H, 2014 (16) | 55.3 | 9.06 | 20 | 59.45 | 15.23 | 57.59 | 14.68 | E | E | E | E | N | ||
| Wang L, 2013 (17) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | N | ||
| Zhang Z, 2012 (18) | 58 | 14 | 30 | 66 | 12 | 72 | 20 | 70 | 12 | 72 | 14 | Y | ||
| El-Ansary M, 2010 (20) Intrasplenic | 35.81 | 4.89 | 6 | NR | NR | 48.93 | 13.06 | E | E | E | E | N | ||
| El-Ansary M, 2010 (20) Peripheral | 41.83 | 16.52 | 6 | NR | NR | 57 | 11.3 | E | E | E | E | N | ||
| Shi M, 2012 (21) | 35 | 4 | 24 | 72 | 20 | 76 | 17 | 82 | 16 | 85 | 14 | Y | ||
| Peng L, 2011 (22) | 26.25 | 5.34 | 53 | 14.82 | 2.53 | 16.23 | 2.56 | 15.64 | 3.17 | 16.32 | 2.97 | N | ||
| El-Ansary, 2012 (23) | 44.3 | 14.7 | 15 | 51.6 | 13.6 | 50 | 15 | E | E | E | E | N | ||
| S.Alb | Amin MA, 2013 (11) | 2.8 | 0.2 | 20 | 2.9 | 0.15 | 3.15 | 0.05 | E | E | E | E | mg/dL | Y |
| Jang YO, 2014 (12) | 3.5 | 0.6 | 11 | NR | NR | 3.9 | 0.5 | E | E | E | E | mg/dL | Y | |
| Kharaziha P, 2009 (13) | 30 | 5 | 8 | NR | NR | 33 | 5 | E | E | E | E | g/L | N | |
| Mohamadnejad M, 2013 (14) | 3.3 | 0.6 | 14 | 3.3 | 0.7 | 3.3 | 0.5 | NR | NR | 3.1 | 0.8 | mg/dL | N | |
| Mohamadnejad M, 2007 (15) | 3.3 | 0.59 | 4 | NR | NR | 3.55 | 0.62 | NR | NR | 3.4 | 0.39 | mg/dL | Y | |
| Salama H, 2014 (16) | 2.59 | 0.28 | 20 | 2.99 | 0.26 | 3.06 | 0.36 | E | E | E | E | mg/dL | N | |
| Wang L, 2013 (17) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | N | |
| Zhang Z, 2012 (18) | 2.8 | 0.7 | 30 | 3.25 | 0.55 | 3.3 | 0.4 | 3.3 | 0.7 | 3.5 | 0.5 | mg/dL | Y | |
| El-Ansary M, 2010 (20) Intrasplenic | 2.2 | 0.12 | 6 | NR | NR | 2.43 | 0.39 | E | E | E | E | mg/dL | N | |
| El-Ansary M, 2010 (20) Peripheral | 2.48 | 0.28 | 6 | NR | NR | 2.76 | 0.28 | E | E | E | E | mg/dL | N | |
| Shi M, 2012 (21) | 31.4 | 2.7 | 24 | 34.7 | 7.0 | 38.2 | 5.9 | NR | NR | 41.8 | 5.3 | g/L | Y | |
| Peng L, 2011 (22) | 29.67 | 3.14 | 53 | 36.75 | 2.27 | 36.93 | 2.43 | 37.5 | 2.31 | 36.83 | 2.18 | g/L | N | |
| El-Ansary, 2012 (23) | 2.3 | 2.0-2.8 | 15 | 2.5 | 2.0-3.0 | 2.6 | 2.0-2.9 | E | E | E | E | mg/dL | N | |
| Amer ME, 2011 (24) | 2.1 | NR | 20 | 2.0 | NR | NR | NR | NR | NR | NR | NR | N | ||
| T.Bil | Amin MA, 2013 (11) | 2.4 | 0.5 | 20 | 2.1 | 0.7 | 1.6 | 0.3 | E | E | E | E | mg/dL | Y |
| Jang YO, 2014 (12) | 1.3 | 0.9 | 11 | NR | NR | 1.1 | 0.7 | E | E | E | E | mg/dL | N | |
| Kharaziha P, 2009 (13) | 2.7 | 1.7 | 8 | NR | NR | 2.41 | 1.82 | E | E | E | E | mg/dL | N | |
| Mohamadnejad M, 2013 (14) | 2.6 | 1.4 | 14 | 4.1 | 2.4 | 2.2 | 1 | NR | NR | 2.2 | 1.5 | mg/dL | N | |
| Mohamadnejad M, 2007 (15) | 2.65 | 1.32 | 4 | NR | NR | 3.12 | 1.59 | NR | NR | 2.51 | 1.12 | mg/dL | Y | |
| Salama H, 2014 (16) | 1.88 | 1.05 | 20 | 1.82 | 1.3 | 2.06 | 1.26 | E | E | E | E | mg/dL | N | |
| Wang L, 2013 (17) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | N | |
| Zhang Z, 2012 (18) | 42 | 22 | 30 | 30 | 17 | 29 | 16 | 28 | 18 | 26 | 18 | µM | Y | |
| Wang L, 2014 (19) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | N | |
| El-Ansary M, 2010 (20) Intrasplenic | 4.3 | 1.73 | 6 | NR | NR | 2.79 | 1.21 | E | E | E | E | mg/dL | Y | |
| El-Ansary M, 2010 (20) Peripheral | 2.86 | 0.86 | 6 | NR | NR | 1.53 | 0.39 | E | E | E | E | mg/dL | Y | |
| Shi M, 2012 (21) | 325 | 124 | 24 | 50 | 50 | 45 | 40 | 28 | 40 | 25 | 10 | µM | Y | |
| Peng L, 2011 (22) | 201.17 | 75.45 | 53 | 27.08 | 6.39 | 22.17 | 4.62 | 27.6 | 10.29 | 26.83 | 5.78 | µM | Y | |
| El-Ansary, 2012 (23) | 5.4 | 1.0-44.4 | 15 | 2.4 | 1.0-10.7 | 0.9 | 0.2-6.9 | E | E | E | E | mg/dL | Y | |
| Amer ME, 2011 (24) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | N | |
| MELD | Jang YO, 2014 (12) | 9.2 | 2.8 | 20 | NR | NR | 8.3 | 2.4 | E | E | E | E | Y | |
| Kharaziha P, 2009 (13) | 17.9 | 5.6 | 8 | NR | NR | 10.7 | 6.3 | E | E | E | E | Y | ||
| Mohamadnejad M, 2013 (14) | 15.4 | 5.1 | 14 | 15.3 | 8.2 | NR | NR | NR | NR | 14 | 3.6 | Y | ||
| Mohamadnejad M, 2007 (15) | 17 | 1.41 | 4 | 13.75 | 4.5 | 13.75 | 4.5 | NR | NR | 15.25 | 3.4 | Y | ||
| Wang L, 2013 (17) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | N | |
| El-Ansary M, 2010 (20) Intrasplenic | 23.33 | 5.95 | 6 | NR | NR | 18.16 | 4.49 | E | E | E | E | Y | ||
| El-Ansary M, 2010 (20) Peripheral | 17 | 3.41 | 6 | NR | NR | 11.33 | 2.16 | E | E | E | E | Y | ||
| Shi M, 2012 (21) | 24.05 | 4 | 24 | 9.2 | 5.8 | NR | NR | NR | NR | NR | NR | Y | ||
| Peng L, 2011 (22) | 29.58 | 1.93 | 53 | 15.29 | 2.25 | 14.67 | 2.89 | 15.55 | 1.73 | 17.39 | 2.68 | Y | ||
| El-Ansary, 2012 (23) | 21.0 | 12.0-38.0 | 15 | 17.0 | 9.0-31.0 | 17.0 | 9.0-26.0 | E | E | E | E | Y | ||
| Amer ME, 2011 (24) | 11.57 | 2.26 | 20 | NR | NR | 11.66 | 2.29 | E | E | E | E | N | ||
| Child | Jang YO, 2014 (12) | 7.1 | 0.9 | 20 | NR | NR | 5.4 | 0.7 | E | E | E | E | Y | |
| Mohamadnejad M, 2013 (14) | 7.7 | 2.5 | 14 | 7 | 2.9 | NR | NR | NR | NR | 7.2 | 1.7 | Y | ||
| Salama H, 2014 (16) | Child A0, B0, C10 | Child A1, B16, C3 | Child A0, B14, C6 | E | E | E | E | Y | ||||||
| Amer ME, 2011 (24) | 11.45 | 1.09 | 20 | NR | NR | 11.45 | 0.95 | E | E | E | E | N | ||
INR, international normalized ratio; PT, prothrombin time; S.Alb, serum albumin; T.Bil, total bilirubin; MELD, model for end-stage liver disease; Child, child-pugh score; M, mean; SD, standard deviation; pre, pretest; post, posttest; No., number of patients; NR, not reported; E, end of study; Y, yes; N, no.
Notes
AUTHOR CONTRIBUTION Conceived and designed the experiments: Kim G, Baik SK. Analyzed the data: Kim G, Shin Y, Lim YL, Kim MY, Kwon SO, Chang SJ. Contributed reagents/materials/analysis tools: Kim G, Baik SK, Shin Y, Chang SJ. Wrote the first draft of the manuscript: Kim G, Eom YW. Wrote the paper: Kim G, Eom YW, Baik SK. ICMJE criteria for authorship read and met: Kim G, Eom YW, Baik SK. Agree with manuscript results and conclusions: Kim G, Eom YW, Baik SK, Kim MY, Kwon SO, Chang SJ.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download