Abstract
Mesenchymal Stromal Cells (MSCs) are potential cellular candidates for several immunotherapy purposes. Their multilineage potential and immunomodulatory properties make them interesting tools for the treatment of various immunological diseases. However, depending on the local microenvironment, diverse biological functions of MSCs can be modulated. Indeed, during infections such as obtained following TLR-agonist engagement (called as TLR priming), the phenotype, multilineage potential, hematopoietic support and immunomodulatory capacity of MSCs can present critical changes, which could further affect their therapeutic potential. Thus, for appropriate clinical application of MSCs, it is important to well know and understand these effects in particular during infectious episodes and to find the suitable experimental settings to study that. Pre-stimulation of MSCs with a specific TLR ligand may serve as an effective priming step to modulate one of its function to achieve a desired therapeutic issue.
Mesenchymal stromal cells (MSCs) represent nowadays an important immunotherapeutic cell population with several possible application for the treatment of immunological-based diseases (1). MSCs are known as multipotent, non-hematopoietic cells that can be found in almost all tissues (2). Despite some common characteristics, source-dependent differences have recently emerged and lead to different clinical applications of MSCs (3). Several reports have shown that differences in the protein and transcriptomic profiles, as well as in the secretome and miRNome of MSCs may reflect differences in their biological properties. From osteogenic stem cell or a bone marrow (BM) stromal cell (4), to Medicinal Signaling Cells as suggested by Caplan and Correa (5), the terminology of MSCs varied across the time. A position statement released by the International Society for Cell Therapy (ISCT) proposed to clarify the nomenclature of MSCs. This statement suggested that the fibroblast-like plastic-adherent cells, regardless of the tissue from which they are isolated, should be termed multipotent mesenchymal stromal cells and thus keeping the acronym MSCs (6). Moreover, to better characterize MSCs and to standardize the research in the field, the ISCT proposed a minimal set of standard criteria to define human MSCs (7). First, during culture MSCs must be plastic-adherent, present a fibroblast like shape and able to give rise to CFU-F (colony forming unit-fibroblasts). Second, based on a flow cytometry analysis of their immunophenotype, MSCs must be positive (>95%) for CD105, CD73 and CD90, and negative (<5%) for CD45, CD34, CD14 (or CD11b), CD79alpha (or CD19) and HLA-DR surface molecules. Third, MSCs must differentiate in vitro to osteoblasts, adipocytes and chondroblasts under specific culture conditions (8). Regarding their therapeutic potential, several properties were associated with MSCs highlighting thus the importance of their use for different therapeutic purposes. Today, it is widely accepted that MSCs are actively involved in the hematopoiesis support (910). MSC are part of the highly specialized “bone marrow microenvironment” and are critical for forming the niche that maintains Hematopoietic Stem Cells (HSCs). MSCs actively participate in the regulation of HSC survival, quiescence and, upon specific triggers, differentiation into mature cells (11) suggesting their role in the enhancement of hematopoietic engraftment during use in HSC transplantation (12). Moreover, it is known that under specific differentiating factors, MSCs could differentiate not only into tissues of mesodermal origin, but also in other tissue lineage cells (1314). A such multilineage potential is an MSC's hallmark allowing their use in regenerative medicine for different repair therapy indications (15). Finally, along with their non-immunogenic state as indicated by the lack of HLA-DR expression, MSCs have the ability to present a potent immunomodulatory potential allowing to regulate both adaptive and innate immunity. This unique feature leads to investigate MSC as a new cellular therapeutic strategy for immune-mediated diseases. Mechanistically, immunomodulation occurs by different pathways but two important sides have to be taken into account: the regulatory network of factors and the gathering of regulatory immune cells. These pathways compete to establish a tolerogenic state conducive for immunomodulation (1617).
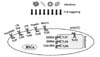
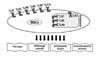
MSCs are also defined as environmentally responsive therapeutics as they are capable of responding to local environmental stimuli with a myriad of beneficial interventions (18). Indeed, MSCs were reported to be particularly sensitive to different environmental signals (19). Infection are known to be major events triggering graft-versus-host disease (GVHD) after allogeneic stem cell transplantation (20). Mimicking infection through Toll-Like Receptor (TLR) activation has been shown to modulate the functions and responses of MSCs (2122). In the following review, we discuss the importance to well study and understand the impact of infections via TLR activation on the biology of MSCs particularly when therapeutic applications have to be proposed. Several MSC biological functions such as phenotype, multilineage potential, hematopoietic support and immunomodulatory capacity have been observed to be drastically affected by specific TLR-agonist engagement (Figs. 1 and 2).
In general, pathogenic infectious agents are detected and destroyed rapidly by the defense mechanisms of innate immunity providing thus the first barrier against pathogens. Host-pathogen interactions are generally initiated when host recognizes conserved molecular structures that are essential for the life-cycle of the pathogen and which are known as a pathogen-associated molecular patterns (PAMPs) (23). PAMPs are sensed by the host's germline encoded pattern recognition receptors (PRRs), expressed by various immune cells such as dendritic cells (DC), macrophages or neutrophils (242526). When PAMPs are recognized by PRRs, an immune response is triggered in the host through activation of a complex signaling pathways which facilitates the eradication of pathogens (25). To date, several classes of PRRs are characterized, and among them Toll-Like Receptors are the most widely studied.
Nowadays, TLRs are considered as the primary sensors of pathogen presence and are involved in the immune response during infections. TLRs are type I transmembrane glycoproteins with extracellular domain rich in leucine repeats that is responsible for PAMPs recognition. The trans-membrane domains, and cytoplasmic Toll-Interleukin 1 Receptor (TIR) domains are required for downstream signaling. Until now, 10 functional human TLR are described (24) depending on their cellular localization and the nature of PAMP ligands that they sense.
TLRs are divided into two sub-groups:
TLRs are functional multimers and most of them are homomeric. However, TLR2, which strictly associates with TLR1 or TLR6 (27) is considered heteromeric.
TLR4 is known to sense lipopolysaccharide (LPS) which is an important component of cell walls of gram-negative bacteria. To be able to bind LPS and to initiate signal transduction, TLR4 forms a complex with accessory molecules such as myeloid differentiation-2 (MD2 also known as Lymphocyte antigen 96), LPS-binding protein subunit (LBP) and CD14 (28). TLR2 senses peptidoglycan, and as a heterodimer with TLR1 (TLR2/TLR1) or TLR6 (TLR2/TLR6) recognizes triacylated and diacylated lipopeptides respectively (2930). TLR3 senses double-stranded RNA (dsRNA), TLR5 senses flagellin protein (31), TLR7 and TLR8 sense RNA and TLR9 senses unmethylated CpG DNA fragments (2632). Despite extensive research on the TLRs, human TLR10 has remained an orphan receptor without a known agonist or function (33).
Although TLRs are primarily thought to have evolved as sensors of exogenous stimuli, the recognition of endogenous ligands is now considered to have an important role in regulating the inflammation. These ligands are called “danger signals” as TLRs can sense them in the setting of injury or non-infectious threat (34). Importantly, TLR activation has been implicated in the pathology of various inflammatory diseases including rheumatoid arthritis or inflammatory bowel disease (IBD), since they can either initiate or perpetuate the chronic inflammation due to the continuous exposure to TLR ligands (353637). However, Fuenzalida et al. (38) has recently shown that short in vitro TLR3 pre-conditioning with poly(I:C) enhances the therapeutic efficacy of UCMSCs (umbilical cord matrix stem cells), which is a major breakthrough for developing improved treatments for patients with inflammatory bowel disease.
TLR signaling is mediated by recruitment of different Toll-Interleukin 1 (IL-1) Receptor (TIR) domain-containing adaptor molecules. Currently, there are four cytosolic adaptor proteins described: MyD88 (myeloid differentiation primary response gene 88), TIRAP (TIR domain-containing adapter protein), TRIF (TIR-domain-containing adapter-inducing interferon-β), and TRAM (TRIF-related adaptor molecule) (2431). Recruitment of these adaptor molecules leads to the activation of various transcription factors such as NFkB, IRF3/7, and MAP kinases to induce the production of inflammatory cytokines and type I interferons (39).
Signaling pathways activated by TLRs can be broadly classified as MyD88-dependent and -independent pathways. TLR4 uses both signaling pathways whereas TLR3 use only MyD88-independent one to activate IRF3 and further to induce type I interferons transcription. All the other TLR use MyD88-dependent signaling to activate NFkB to induce transcription of pro-inflammatory cytokines (40).
TLRs are widely expressed by the main cells of the innate and adaptive immune system (414243). TLR expression is not just a feature of immune cells as other cell types such as fibroblasts, epithelial, endothelial and MSCs may express several TLRs and thus contribute to the protection against infection (4445). The first evidence that MSC express functional TLRs comes from the studies done in mice (46). Pevsner-Fisher et al. showed that mouse MSCs express functional TLR molecules 1 to 8, but not TLR-9. In contrast, human MSCs expressed mRNA of different TLRs. While the data concerning the expression of TLR1, TLR2, TLR3, TLR4, TLR5 and TLR6 are quite consistent, the expression of TLR7, TLR8, TLR9 and TLR10 is quite controversial and it seems that is MSC-origin dependent (21474849505152). As demonstrated by Raicevic et al., several environmental conditions modulate the pattern and function of TLRs expressed by MSCs (5354). BM-MSCs and adipose tissue (AT-MSCs) shared the same TLR pattern featured with the transcription of TLR1, TLR2, TLR3, TLR4, TLR5, TLR6 and TLR9 and the absence of mRNA for TLR7, TLR8 and TLR10. Of special interest, MSCs isolated from the umbilical cord matrix, called Wharton's Jelly (WJ-MSCs) do not expressed TLR4 and expressed a nonfunctional TLR3. Cord blood derived MSCs (CB-MSCs), expressed low levels of TLR1,3,5,9 and high levels of TLR4 and TLR6. Protein studies revealed that both TLR4 and TLR5 were functional (55). Expression of TLR1-10 genes was confirmed in human amnion mesenchymal cells (AMC) but with different levels (56). Interestingly, TLR6 and TLR9 were expressed at the highest levels while the expression of the resting isoforms was very low. The relatively low expression and function of some TLRs may be characteristic for MSCs originating from the early developmental stages. Dental MSCs were showed to express mRNA encoding for TLR3 and TLR4 (57). Moreover, hypoxia significantly increased mRNA of TLR1, 2, 5, 9, and 10 (58) whilst infection of MSCs with baculoviral vectors upregulated and activated TLR3 signaling pathway (59). Recent study has shown that the antibacterial effect of MSCs against Escherichia coli was mediated by secretion of β-defensin 2 via TLR4 signaling (60). Lastly, gingival margin-derived stem/progenitor cells (G-MSCs) showed a distinctive TLR expression profile. Constitutively, G-MSCs expressed TLRs 1, 2, 3, 4, 5, 6, 7, and 10. Inflammation significantly up-regulated TLRs 1, 2, 4, 5, 7 and 10 and diminished TLR 6 expression (61). Collectively, an inflammatory environment may modulate the pattern and function of TLRs expressed by MSCs depending on their tissue source origin.
Within the immune system, TLR activation can modulate the expression of Human leucocytes antigens (HLA) as well as several costimulatory molecules thus impairing the immunological status and function of immune cells. Therefore, it is of great relevance regarding their use in allogeneic cell-based therapies to determine whether MSC exposure to TLR ligands may induce the expression of HLA-I, HLA-II, and costimulatory molecules (CD40, CD80, CD86). Even in the presence of IFN-γ (a well-known immunological inducer), activation of TLR4, TLR3 and TLR2 with LPS, Poly I:C, and PGN respectively had no significant effects on the immunogenic properties of MSCs from BM and AT (474962636465). Indeed, except induction of HLA-I by Poly I : C, none of the other ligands were able to alter the expression of HLA-II, CD80, and CD86. However, another study demonstrated adverse effects of TLR3 and TLR4 activation on the immunological phenotype of umbilical cord derived mesenchymal stem cells (UC-MSCs). They found that the expression of co-stimulatory proteins CD80 and CD86 were unaffected following treatment with the TLR3 agonist (Poly I : C). In the other side, only TLR4 ligand (LPS) increased the expression of CD86. Interestingly, the expression of CD74 (HLA class II histocompatibility antigen gamma chain also known as HLA-DR antigens-associated invariant chain) and CD105 was not modulated by TLR activation (6667).
It appears that TLRs and their ligands can serve as regulators of MSC immunophenotype and might affect the maintenance of their immunogenicity state.
The multilineage potential of MSCs is considered to mediate their therapeutic effects during tissue repair underlying their importance in regenerative medicine (68). Different studies have reported contrasting effects of TLR activation on MSC multilineage potential with promoting versus altering differentiation capacities.
TLRs have been shown to be differently involved in regulating the differentiation of CB-MSCs (69). Thus, adipogenic differentiation was not altered by TLR activation. TLR2 activation with Pam(3)CSK(4) and TLR4 activation with LPS were able to promote either chondrogenesis and osteogenesis of of CB-MSCs but with different intensities. In another study, the activation of TLR3 by Poly(I:C) inhibited the differentiation of UC-MSCs into osteocytes, while that of TLR4 by LPS increased this differentiation to a certain extent (66). In contrast, even expressing high levels of functional TLRs 3 and 4, the differentiation potential of BM-MSCs was not altered after TLR ligation with their respective ligands (64). However, a recent study demonstrated that TLR4 activation by its ligand LPS promoted the osteogenic differentiation of BM-MSC through Wnt3a and Wnt5a signaling (70). Both TLR-3 activated (Poly(I:C)) and TLR-4 activated (LPS) were reported to promote osteogenesis by favoring the differentiation of BM-MSCs to osteoblasts (71). However, these two TLR sub-types played different roles in different stages of BM-MSC osteogenesis. Zhang and colleagues found that triggering receptors expressed on myeloid cells receptors (TREM-2) is constitutively expressed in BM-MSCs and that TREM-2 knockdown resulted in downregulation of several TLR expression and inhibited osteogenic, chondrogenic, and adipogenic differentiation (72). Although the signaling mechanisms remain unclear, these results strongly suggest that TREM-2 activation is actively involved in the regulation of MSC multipotential potential. PGN (TLR2) and LPS (TLR4)-induced osteogenic differentiation has been demonstrated in stromal cells derived from human adipose tissue (hADSCs) following activation of NF-κB and subsequent up-regulation of PDZ-binding motif (TAZ) expression (58). Lombardo and colleagues (47) showed that TLR3 (Poly I:C) and TLR4 (LPS) ligation on hADSCs increased their osteogenic differentiation potential without affecting the adipogenic one. In parallel, an increased osteogenic differentiation of hADSCs after activation of TLR4 (by its ligand LPS) and TLR2 (by its ligand PGN) is observed in a dose-dependent manner and accompanied by increased ERK activation. Activation of TLR9 (by its ligand CpG-ODN/2216) inhibited osteogenesis capacity of hADSCs whereas TLR3 activation (by its ligand Poly(I:C)) and TLR5 (by its ligand flagellin) had no effect on it (21). In the case of adipogenic differentiation, activation of TLR2 (by its ligand PGN) inhibited it significantly, but the other agonists did not affect it. In addition, Mo et al. found that prolonged LPS challenge for TLR4 activation up-regulated the osteogenic differentiation of human BM-MSCs in contrast to TLR2 (challenged with lipoteichoic acid) that had no effect (73). Furthermore, Pevsner-Fischer et al. (46) showed that TLR signaling may play a distinctive role in regulating mouse BM-MSC multipotency depending on the spontaneous versus induced differentiation lineages that are studied. In non-induced MSC culture, Pam3Cys activating TLR2 promoted spontaneous osteogenic differentiation of MSCs and at the same time inhibited adipogenic differentiation. In MSC cultures induced to differentiate into the three mesodermal lineages, TLR2 activation by Pam3Cys significantly reduced mouse BM-MSC differentiation into osteoblasts, adipocytes, and chondrocytes. Although stimulation with the TLR3 ligand (Poly(I:C)) promoted the differentiation of mouse BM-MSCs into the adipocytes and osteoblasts, stimulation with the TLR4 ligand (LPS) induced the reverse effect as it inhibited this process. The transcription factor NF-κB, which is triggered by the TLR4-MyD88-dependent pathway was activated and involved in the inhibition of MSCs mesodermal differentiation (74). In a comparative study, TLR ligation was reported to differentially affect the osteogenic potential of human MSCs depending on their tissue origin (75). Indeed, TLR3 (Poly(I:C)) or TLR4 (LPS) triggering increased the osteogenesis in hADSCs and, to lesser extent, in BM-MSCs. However, WJ-MSCs constitutively disclosed a lower osteogenic potential as compared with other MSCs, which is not affected by TLR.
It appears that TLRs and their ligands can serve as regulators of MSC differentiation abilities and might affect the maintenance of their multipotency state.
MSCs play an important role in the physiology and homeostasis of the hematopoietic system (76). MSCs support hematopoiesis by creating a niche where HSCs can proliferate and differentiate. Because MSCs generate most of the stromal cells present in the niche, and produce various molecules regulating hematopoiesis, their hematopoiesis-supporting capacity has become increasingly important in the treatment of hematologic malignancies during hematopoietic stem cell transplantation (77). Resident mouse BM-MSCs by producing MCP-1 in response to TLR4 activation by LPS was reported to induce monocyte emigration from bone marrow into circulation to confront potential infections (78). Brümmendorf and colleagues (79) showed that in addition to enhanced myeloid colony formation from human CD34 positive cells, TLR4 stimulation by LPS retains overall higher numbers of CD34+ cells in coculture assays using BM-MSCs, with eightfold more CD34+ cells that underwent up to three divisions as compared to non-stimulated assays. Moreover, CD34+ cells from LPS-stimulated BM-MSC cultures give rise to the full spectrum of myeloid and lymphoid colonies, thus supporting maintenance of primed hematopoietic progenitor cells (HPCs) under inflammatory conditions and TLR4 stimulation (79). In BM-MSCs, TLR2 (PAM(3) CSK(4)) and TLR4 (LPS) activation by their respective agonist increased their production of hematopoiesis-related cytokines promoting thus the proliferation and the differentiation of CD34+ cells (52).
Recent study by Iwamura et al. (80) shows that recognition of commensal-derived PAMP by NOD1, but not NOD2, induced expression of multiple hematopoietic cytokines (IL-7, FMS-like tyrosine kinase 3 ligand (Flt3L), stem cell factor (SCF), ThPO, and IL-6) from BM-MSCs indicating that NOD1 signaling in MSCs serves as an important pathway underlying the requirement for microbiota in the maintenance of steady-state hematopoiesis.
Based on the results in this study, TLR4 (LPS) or NOD1 ligand-stimulated BM-MSCs are both likely to promote hematopoiesis. LPS as a potent inducer of IL-6 favors myelopoiesis, whereas NOD1 ligand augments the numbers of all HSPCs (80).
It appears that little observations are available for the impact of TLRs on MSC hematopoiesis support.
Besides not being recognized as immunogenic, MSCs can actively sense their surrounding microenvironment and accordingly regulate the function and biology of different immune cells (81). Thus, MSCs have the capacity to interact with different immune cells from both innate and adaptive system and to induce their modulation.
To achieve their desired effects, MSCs have to be recruited to sites of injuries where they will display the functions. Stimulation of TLR present on BM-MSC activated downstream signaling pathways with the greatest activation observed for TLR3 (Poly(I:C)). Consequently, and in a TLR-ligand depending manner, this triggering induced the secretion of cytokines and chemokines mainly involved in cell migration (48). Indeed, MSC migration was critically promoted by TLR ligand exposure with TLR3 as primarily mediating the stress migration responses of MSCs when compared to TLR2 and TLR9. In line, TLR3 (Poly I : C) and TLR4 (LPS) triggering have thus converted BM-MSCs into powerfully chemotactic cells capable of enhancing recruitment of inflammatory immune cells by increasing their production of IL-1β, IL-6, IL-8, CCL5 (RANTES), IP10 and monocyte chemotactic protein (MCP)-1 via activation of NF-kB signaling (51). Similar results have been obtained in AT-MSC, where TLR agonists (PGN for TLR2 and LPS for TLR4) increased mRNA production of MCP-1 and -2, granulocyte chemotactic protein-2 (GCP-2), IL-1β, macrophage inflammatory protein-3α (MIP-3α) (58). Human turbinated MSC (hTMSC) were shown to express relatively high percentage of TLR3 and TLR4. However, hTMSCs were only immunologically active and responsive to TLR4 as demonstrated by the substantial changes in their cytokine and chemokine profiles (82). Macrophage-activating ligand-2 (MALP-2), agonist of TLR6 and its known heterodimer partner TLR2, induced activation of NF-κB pathway and lead AMC to acquire a pro-inflammatory cytokine profile by highly secreting IL-4, IL-6, and IL-8 (56).
Unlike TLR3 (poly I:C), activation of TLR4 with LPS strongly and significantly induced expression of IL-6, IL-8, IL-12, IP-10 (CXCL10), RANTES (CCL5), TNF-α, and GM-CSF. In parallel, Tomchuck et al, (83) demonstrated that upon TLR3 stimulation (Poly(I:C)), a Janus kinase (JAK) 2/signal transducer and activator of transcription (STAT) 1 pathway is activated, and the expression of suppressor of cytokine signaling (SOCS) proteins is increased. These results further demonstrated that SOCS1 and SOCS3 play a distinct role in negatively modulating TLR3, JAK/STAT, and CXCR4/CXCR7 signaling in BM-MSCs. Collectively, these data suggest that as negative regulators, SOCS proteins critically affect the way MSC respond to danger signals. These observations suggest that TLR signaling pathways may be manipulated to increase the bio-distribution of infused MSCs at the injured sites.
Migration and binding of immune cells to MSCs surrounding environment has been reported to be a key step for establishing immunomodulation (84). Although TLR4 activation with LPS elicited the secretion of pro-inflammatory mediators CXCL1, IL-6, IL-8, and CCL2, Poly (I:C) activating TLR3 increased only the secretion of IL-6 and MIF (macrophage migration inhibitory factor) known to be important in leucocyte recruitment (63). Upon challenge with different TLR ligands on MSCs isolated from human nasal mucosa (nmMSCs), only activation of TLR3 with its Poly I:C ligand induced the strongest release of proinflammatory cytokines (IL-6 and IL-8) and type I interferon (85). Moreover, under TLR3 stimulation, mesenchymal stromal cells from human tonsils (T-MSCs) acquire a chemoattractant profile that is suitable for allowing immune cells to migrate into MSCs surrounding environment. Indeed, TLR3 activation increased in the secretion of many chemokine such as CXCL5, CXCL6, CXCL1, CXCL8, and CXCL10 (67). In terms of leukocyte binding, BM-MSCs responded differently to TLR3 and TLR4 activation (63). TLR3 pre-activation with Poly I:C significantly increased the number of leukocytes that bind to MSCs, predominantly through interacting with hyaluronic acid structures whereas activation of TLR4 with LPS increased VCAM-1 and ICAM-1 dependent binding of leukocytes to MSCs.
The expression of B cell activating factor (BAFF), a member of the tumor necrosis factor ligand superfamily with notable stimulating activity on B cells was investigated in BM-MSCs from both human and murine species. The BAFF expression was increased in the presence of TLR4 agonist (LPS), while TLR2 agonist (Zymosan) and TLR3-agonist (poly I:C) had no effect. These results suggested that TLR4 and downstream pathways in MSCs exert an important function in B lymphocyte-related immune regulation (86).
Once the immune cells are in the surrounding area of MSCs, different regulatory mechanisms can take part in the process of immunomodulation. These mechanisms are actively sensitive to the environment of MSCs and thus tightly regulated to allow adequate and efficient response of MSCs. Within the literature, the results about TLR priming and immunomodulation are conflictual and reported in very different ways.
Waterman et al. reported a new paradigm for MSC immunomodulation functions as they can be specifically polarized by downstream TLR signaling into two homogenously acting types (65). Indeed, TLR4-primed MSCs (MSC1), mostly producing pro-inflammatory mediators (MIP-1α and MIP-1β, RANTES, CXCL9 and CXCL10), are able to induce T-lymphocyte activation, while TLR3-primed MSCs (MSC2), mainly expressing immunosuppressive factors : IDO (indoleamine-2,3-dioxygenase), PGE2 (prostaglandin E2), NO (nitric oxide), TGF-β, Hepatocyte Growth Factor (HGF) and hemoxygenase (HO), lead to T-cell inhibition. Levin and colleagues (87) investigated the molecular basis for the heterogeneity in the response of MSCs to TLR activation. They found that divergent levels of LPS binding protein (LBP) lead to the heterogenic response of murine BM-MSCs to TLR activation. In the TLR signaling pathway, LBP levels predicted the ability of specific MSCs to secrete pro-inflammatory cytokines in response to LPS.
Although some studies reported no significant effect of TLR activation on AD-MSC, BM-MSC and T-MSC-mediated immunosuppression (4782), others reported mitigated observations. Indeed, differently activated MSCs isolated from human nasal mucosa (nmMSCs) either by TLR3 (poly I:C) or TLR4 (LPS) maintained their ability to suppress leukocyte activation at similar levels, and this effect was shown to be partially mediated by prostaglandins [60]. Liotta et al., demonstrated that ligation of TLR3 (poly I:C) and TLR4 (LPS) by their respective ligands impaired the ability of human BM-MSCs to suppress the proliferation of T-cells (64). These effects were not associated with alteration of IDO and PGE2 pathways known to be the main mediators of MSC immunosuppression but rather involved jagged-1 down-regulation induced by TLR3 or TLR4 ligation. Indeed, strong evidences indicate that the Jagged-1/Notch interaction may be involved in the suppressive activity of MSCs on T-cell proliferation.
In contrast, Opitz and colleagues reported that TLR3 (poly I:C) and TLR4 (LPS) engagement enhanced the suppressive properties of human BM-MSCs as shown by the increase in their production of regulatory kynurenines by the tryptophan-degrading enzyme IDO1 (49). Induction of IDO1 by TLR involved an autocrine interferon (IFN)-beta signaling loop, which was dependent on protein kinase R (PKR), but independent of IFN-gamma. In a comparative study, TLR3 (poly I:C) and TLR4 (LPS) ligation have differentially affected the suppressive functions of BM-, WJ- and AT-MSCs (54). Remarkably, the immunosuppressive potential of WJ- and AT-MSC was not affected while BM-MSC showed reduced ability to inhibit lymphocyte activation. Differences in the levels of HGF and PGE2 secreted by MSCs following TLR activation have been hypothesized to underline these changes. As shown by Lei J. et al. (88), ligation of TLR2 (Pam3Cys) and TLR4 (LPS) on BM-MSCs could trigger differential effects on their immunosuppressive activity. Interestingly, TLR2 but not TLR4 activation significantly impaired MSC-mediated immunosuppression of T-cells and reduced MSC-mediated expansion of CD4+CD25+Foxp3+ regulatory T cells. CXCL10 and iNOS expression and modulation by BM-MSCs following TLR activation have proposed to mediate these effects but should be further clarified. Interestingly, it has been suggested that during bacterial infection, mouse BM-MSCs may retain a reservoir of the TLR2 ligands (Pam3Cys), in a long-term manner, and release them slowly to maintain an immune response (89). Pam3Cys was transferred from cultured MSCs to immune cells which induced a pro-inflammatory response in vitro and in vivo. In contrast, Rashedi et al. (90) found that the generation of Tregs from human lymphocyte cultures was enhanced by either TLR3 (poly I:C) or TLR4 (LPS) activation of MSCs. This Treg induction was associated with increased gene expression of the Notch ligand, Delta-like and suggested a new way to enhance the immunomodulatory functions of MSCs. Activation of TLR4 (LPS) and TLR5 (flagellin) in CB-MSCs do not affected their immunosuppressive activity (55). However, such activation impaired the cytokine secretion profile of unrestricted somatic stem cells (USSCs) independently of TLR ligand nature. Indeed, stimulation of USSCs with either LPS or flagellin resulted in a marked increase of IL-6 and/or IL-8 production although levels differed significantly between both stimuli. Stimulation with TLR2 (PAM3CSK4) or TLR4 (LPS) induced a marked expression change in the microRNAs profile of BM-MSCs (91). The potential target genes of these microRNAs were suggested to be involved in the regulation of BM-MSC functions. Another study reported that, after treating CB-MSCs with various TLR ligands, only TLR3 ligand, poly(I:C), significantly improved their immunosuppressive abilities by increasing the expression of cyclooxygenase-2 (COX-2) known to be the key enzyme in PGE2 production (92). Subsequent results indicated that miR-143 regulates the effect of poly(I:C) on MSC immunosuppressive function by targeting COX-2 gene.
Data from the literature have previously highlighted that MSC-mediated T-cell suppression occurs through the secretion of galectins. In response to TLR2 activation (PGN), the expression of galectin-3, known to modulate T-cell biology, was up-regulated at both mRNA and protein levels (93) in BM-MSCs. However, such up-regulation was not linked to a change in MSC immunomodulatory response. Moreover, Gieseke F. et al. (94) demonstrated that galectin-9, which is not constitutively expressed by BM-MSCs, is strongly induced upon interaction with inflammatory cells and functionally important for the immunosuppressive effects of MSCs. Indeed, galectin-9 expression differentially induced by BM-MSCs depending on the TLR ligand activation. Induction of galectin-9 was observed following TLR2 (zymosan), TLR3 (Poly(I:C)) and TLR4 (LPS) activation. In contrast, TLR5 (flagellin) and TLR7/8 (R-848, imidazolquinoline compound) did not show any effects on galectin-9 expression. Thus, in the presence of a specific infectious stimuli (TLR activation), BM-MSCs maintain their immunosuppressive by inducing the expression of galectin-9. In the other hand, the immunomodulatory properties of dental pulp (DP) and dental follicle (DF)-MSCs were shown to be differently modulated by TLR ligation (57). Activation of TLR3 with Poly(I:C) augmented the suppressive potential of both cell types by potentiating their TGF-β and IL-6 secretion. In contrast, TLR4 activation with LPS increased the suppressive effect of DF-MSCs by enhancing their TGF-β production but abrogated that of DP-MSCs by inhibiting their TGF-β production and IDO-1 expression. These contrasting effects were suggested to be correlated with the higher expression of TLR3 and TLR4 in DP-MSCs compared with DF-MSCs. Additionally, activation of TLR3 with Poly(I:C) enhanced the suppressive functions of T-MSCs against Th17 differentiation by increasing programmed death ligand-1 (PD-L1) expression (95).
Moreover, TLR3 preconditioning by Poly(I:C) have been recently demonstrated to enhance the therapeutic efficacy of UC-MSCs via the TLR3-Jagged-1-Notch-1 pathway (96). Poly (I:C)-MSCs showed enhanced suppressive effects in vitro and in vivo through increasing production of PGE2 and up-regulation of Jagged-1. Furthermore, PGE2 subsequently increased the secretion of IL-10 and promoted the differentiation of Treg. In addition, TLR activation has been reported to influence the cytokine balance and thereby controls the outcome of T-cell-mediated response (97). As stated Raicevic et al, (53), TLR activation may affect MSCs immunomodulatory functions by modulating their cytokine profile. Indeed, a decrease in the immunosuppressive capabilities of BM-MSCs is observed following TLR3 and TLR4 activation by poly(I:C) and LPS respectively. Moreover, TLR3 activation augmented IL-6, IL-12p35, IL-23p19, and IL-27p28 transcription, whereas TLR4 activation increased IL-23p19 and IL-27p28 transcription. These IL-12 cytokine family members are known to drive CD4+ T helper 1 (TH1) differentiation and thus promote a T-cell-mediated inflammatory response. TLR3 and TLR4 triggering induced a pro-inflammatory shift in the cytokine profile of BM-MSCs that should be associated with their reduced immunomodulatory functions.
As mentioned in the introduction, MSCs have also immunomodulatory effects toward cells of the innate immune response. Data from Cassatella and colleagues revealed that TLR3- and TLR4-activated MSCs differently prolonged the survival and function of neutrophils (PMN) (98). Results have showed that TLR3 triggering by poly(I:C) dramatically amplifies, in a more significant manner than TLR4 triggering by LPS, the anti-apoptotic effects of activated MSCs in comparison to resting BM-MSCs on PMN. In addition, TLR3- and TLR4-activated BM-MSCs enhanced the respiratory burst ability and CD11b expression by PMN. The biological effects exerted on PMN by TLR3-activated BM-MSCs were mediated by the combined action of IL-6, IFN-β, and GM-CSF, while those exerted by TLR4-activated BM-MSCs were mostly depended on GM-CSF. MSCs and NK cells have been described to interact in a complex manner with bidirectional regulatory effects. Although they are able to alter most of activated NK cell biology, MSCs are reported to be susceptible to NK-cell mediated lysis (17). The results of Giuliani M. et al, showed that TLR3-primed (poly(I:C)) BM-MSCs were more resistant than unprimed MSCs to IL-2-activated NK-induced killing (99). In contrast, no potentialized protection was observed after TLR4 or TLR7/8 priming of BM-MSCs. Such protection can be explained by the modulation of Natural Killer group 2D ligands major histocompatibility complex class I chain A and ULBP3 and DNAM-1 ligands by TLR-primed MSC. In addition, TLR3-primed MSC enhance their suppressive functions against NK cells. Furthermore, activation of TLR4 pathway by LPS ligand has demonstrated more MSC suppressive effect towards NK cell proliferation and cytotoxicity and thus may provide a potential stroma-targeted tumor therapy (100). Thus, TLR-primed MSCs are able to adapt their immuno-behavior in an inflammatory context by decreasing their susceptibility to NK killing and by enhancing their immunosuppressive abilities.
It appears that TLRs and their ligands can serve as regulators of MSC immunomodulatory capacities but the effects are divergent and likely depending on experimental settings.
Understanding the effects of TLR activation on MSCs immunobiology is of great importance to allow efficient use of their therapeutic effects. In this review, we discussed and compared the results underlying the relationship between MSCs and activation of different TLRs. We focused on the fact that TLRs priming could critically influence the phenotype, multilineage potential, hematopoietic support and immunomodulation capacity of MSCs as they are the main properties of these therapeutic cells. Despite the large amount of data obtained, there is great discrepancy of results among the studies. These differences are probably related to the diversity of experimental settings that are used to study the impact of TLRs on MSCs. In particular, we highlight the important influence of specific culture conditions (e.g. medium, cell ratio), MSC origin (murine or human), MSC source (e.g. bone marrow, cord blood, adipose tissue, etc…), TLR target (e.g. TLR1,2,3, etc…), TLR ligation characteristics (e.g. type of ligand, concentration, duration, etc…), and the end points to be achieved. Thus, in order to allow safer and more efficient therapeutic use of MSCs, the impact of infectious conditions and thus TLR activation have to be critically studied in well-designed and standardized assays. Pre-stimulation of MSCs with a specific TLR ligand may serve as an effective priming step to modulate one of its function to achieve a desired therapeutic issue.
Figures and Tables
References
1. Fayyad-Kazan M, Fayyad-Kazan H, Lagneaux L, Najar M. The potential of mesenchymal stromal cells in immunotherapy. Immunotherapy. 2016; 8:839–842.

2. Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011; 9:12.

3. El Omar R, Beroud J, Stoltz JF, Menu P, Velot E, Decot V. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng Part B Rev. 2014; 20:523–544.

4. Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988; 136:42–60.

6. Horwitz EM, Le BK, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005; 7:393–395.

7. Dominici M, Le BK, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317.

8. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.

9. Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014; 35:32–37.

10. Fajardo-Orduna GR, Mayani H, Montesinos JJ. Hematopoietic support capacity of mesenchymal stem cells: Biology and clinical potential. Arch Med Res. 2015; 46:589–596.

11. Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013; 31:285–316.

12. Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009; 11:503–515.

13. Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007; 25:1384–1392.

14. De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003; 174:101–109.

15. Kariminekoo S, Movassaghpour A, Rahimzadeh A, Talebi M, Shamsasenjan K, Akbarzadeh A. Implications of mesenchymal stem cells in regenerative medicine. Artif Cells Nanomed Biotechnol. 2016; 44:749–757.

16. Najar M, Raicevic G, Crompot E, Fayyad-Kazan H, Bron D, Toungouz M, Lagneaux L. The immunomodulatory potential of mesenchymal stromal cells: A story of a regulatory network. J Immunother. 2016; 39:45–59.
17. Najar M, Raicevic G, Fayyad-Kazan H, Bron D, Toungouz M, Lagneaux L. Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy. 2016; 18:160–171.

18. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013; 45:e54.

19. Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007; 28:219–226.

20. Reddy P, Ferrara JL. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003; 17:187–194.

21. Cho HH, Bae YC, Jung JS. Role of toll-like receptors on human adipose-derived stromal cells. Stem Cells. 2006; 24:2744–2752.

22. Pevsner-Fischer M, Zipori D. Environmental Signals Regulating Mesenchymal Progenitor Cell Growth and Differentiation. In : Ms. MPhil VKR, Vemuri MC, editors. Stem Cell Biology and Regenerative Medicine. Regulatory Networks in Stem Cells. Humana Press;2009. p. 175–184.
24. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–384.

25. Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007; 449:819–826.

26. Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011; 30:16–34.

27. Rich T. Toll and Toll-Like Receptors:: An Immunologic Perspective. Springer Science & Business Media;2007. p. 25–26.
29. Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007; 130:1071–1082.

30. Kang JY, Nan X, Jin MS, Youn SJ, Ryu YH, Mah S, Han SH, Lee H, Paik SG, Lee JO. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity. 2009; 31:873–884.

31. Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009; 420:1–16.

33. Guan Y, Ranoa DR, Jiang S, Mutha SK, Li X, Baudry J, Tapping RI. Human TLRs 10 and 1 share common mechanisms of innate immune sensing but not signaling. J Immunol. 2010; 184:5094–5103.

34. Zanin-Zhorov A, Cohen IR. Signaling via TLR2 and TLR4 directly down-regulates T cell effector functions: The regulatory face of danger signals. Front Immunol. 2013; 4:211.

35. Huang Y, Chen Z. Inflammatory bowel disease related innate immunity and adaptive immunity. Am J Transl Res. 2016; 8:2490–2497.
36. Ishihara S, Rumi MA, Ortega-Cava CF, Kazumori H, Kadowaki Y, Ishimura N, Kinoshita Y. Therapeutic targeting of toll-like receptors in gastrointestinal inflammation. Curr Pharm Des. 2006; 12:4215–4228.

37. Yamamoto-Furusho JK, Podolsky DK. Innate immunity in inflammatory bowel disease. World J Gastroenterol. 2007; 13:5577–5580.

38. Fuenzalida P, Kurte M, Fernandez-O'ryan C, Ibanez C, Gauthier-Abeliuk M, Vega-Letter AM, Gonzalez P, Irarrazabal C, Quezada N, Figueroa F, Carrion F. Toll-like receptor 3 pre-conditioning increases the therapeutic efficacy of umbilical cord mesenchymal stromal cells in a dextran sulfate sodium-induced colitis model. Cytotherapy. 2016; 18:630–641.

39. Parker LC, Prince LR, Sabroe I. Translational mini-review series on Toll-like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clin Exp Immunol. 2007; 147:199–207.

40. Krishnan J, Selvarajoo K, Tsuchiya M, Lee G, Choi S. Toll-like receptor signal transduction. Exp Mol Med. 2007; 39:421–438.

41. Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004; 5:987–995.

43. Vaknin I, Blinder L, Wang L, Gazit R, Shapira E, Genina O, Pines M, Pikarsky E, Baniyash M. A common pathway mediated through Toll-like receptors leads to T- and natural killer-cell immunosuppression. Blood. 2008; 111:1437–1447.

44. Hornef MW, Bogdan C. The role of epithelial Toll-like receptor expression in host defense and microbial tolerance. J Endotoxin Res. 2005; 11:124–128.

45. Yang X, Coriolan D, Murthy V, Schultz K, Golenbock DT, Beasley D. Proinflammatory phenotype of vascular smooth muscle cells: role of efficient Toll-like receptor 4 signaling. Am J Physiol Heart Circ Physiol. 2005; 289:H1069–H1076.

46. Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007; 109:1422–1432.

47. Lombardo E, DelaRosa O, Mancheno-Corvo P, Menta R, Ramirez C, Buscher D. Toll-like receptor-mediated signaling in human adipose-derived stem cells: implications for immunogenicity and immunosuppressive potential. Tissue Eng Part A. 2009; 15:1579–1589.

48. Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES, Scandurro AB. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008; 26:99–107.

49. Opitz CA, Litzenburger UM, Lutz C, Lanz TV, Tritschler I, Koppel A, Tolosa E, Hoberg M, Anderl J, Aicher WK, Weller M, Wick W, Platten M. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells. 2009; 27:909–919.

50. Krampera M, Sartoris S, Liotta F, Pasini A, Angeli R, Cosmi L, Andreini A, Mosna F, Bonetti B, Rebellato E, Testi MG, Frosali F, Pizzolo G, Tridente G, Maggi E, Romagnani S, Annunziato F. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. 2007; 16:797–810.

51. Romieu-Mourez R, Francois M, Boivin MN, Bouchentouf M, Spaner DE, Galipeau J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol. 2009; 182:7963–7973.

52. Wang X, Cheng Q, Li L, Wang J, Xia L, Xu X, Sun Z. Toll-like receptors 2 and 4 mediate the capacity of mesenchymal stromal cells to support the proliferation and differentiation of CD34+ cells. Exp Cell Res. 2012; 318:196–206.

53. Raicevic G, Rouas R, Najar M, Stordeur P, Boufker HI, Bron D, Martiat P, Goldman M, Nevessignsky MT, Lagneaux L. Inflammation modifies the pattern and the function of Toll-like receptors expressed by human mesenchymal stromal cells. Hum Immunol. 2010; 71:235–244.

54. Raicevic G, Najar M, Stamatopoulos B, De BC, Meuleman N, Bron D, Toungouz M, Lagneaux L. The source of human mesenchymal stromal cells influences their TLR profile as well as their functional properties. Cell Immunol. 2011; 270:207–216.

55. van den Berk LC, Jansen BJ, Siebers-Vermeulen KG, Netea MG, Latuhihin T, Bergevoet S, Raymakers RA, Kogler G, Figdor CC, Adema GJ, Torensma R. Toll-like receptor triggering in cord blood mesenchymal stem cells. J Cell Mol Med. 2009; 13:3415–3426.

56. Sato BL, Collier ES, Vermudez SA, Junker AD, Kendal-Wright CE. Human amnion mesenchymal cells are pro-inflammatory when activated by the Toll-like receptor 2/6 ligand, macrophage-activating lipoprotein-2. Placenta. 2016; 44:69–79.

57. Tomic S, Djokic J, Vasilijic S, Vucevic D, Todorovic V, Supic G, Colic M. Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. Stem Cells Dev. 2011; 20:695–708.

58. Cho HH, Shin KK, Kim YJ, Song JS, Kim JM, Bae YC, Kim CD, Jung JS. NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol. 2010; 223:168–177.
59. Chen GY, Shiah HC, Su HJ, Chen CY, Chuang YJ, Lo WH, Huang JL, Chuang CK, Hwang SM, Hu YC. Baculovirus transduction of mesenchymal stem cells triggers the toll-like receptor 3 pathway. J Virol. 2009; 83:10548–10556.

60. Sung DK, Chang YS, Sung SI, Yoo HS, Ahn SY, Park WS. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta-defensin-2 via toll-like receptor 4 signalling. Cell Microbiol. 2016; 18:424–436.

61. Fawzy-El-Sayed K, Mekhemar M, dam-Klages S, Kabelitz D, Dorfer C. TlR expression profile of human gingival margin-derived stem progenitor cells. Med Oral Patol Oral Cir Bucal. 2016; 21:e30–e38.

62. DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflamm. 2010; 2010:865601.

63. Kota DJ, DiCarlo B, Hetz RA, Smith P, Cox CS Jr, Olson SD. Differential MSC activation leads to distinct mononuclear leukocyte binding mechanisms. Sci Rep. 2014; 4:4565.

64. Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008; 26:279–289.

65. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010; 5:e10088.

66. Zhang L, Liu D, Pu D, Wang Y, Li L, He Y, Li Y, Li L, Qiu Z, Zhao S, Li W. The role of Toll-like receptor 3 and 4 in regulating the function of mesenchymal stem cells isolated from umbilical cord. Int J Mol Med. 2015; 35:1003–1010.

67. Ryu JH, Park M, Kim BK, Ryu KH, Woo SY. Tonsil-derived mesenchymal stromal cells produce CXCR2-binding chemokines and acquire follicular dendritic cell-like phenotypes under TLR3 stimulation. Cytokine. 2015; 73:225–235.

68. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006; 98:1076–1084.

69. Kim HS, Shin TH, Yang SR, Seo MS, Kim DJ, Kang SK, Park JH, Kang KS. Implication of NOD1 and NOD2 for the differentiation of multipotent mesenchymal stem cells derived from human umbilical cord blood. PLoS One. 2010; 5:e15369.

70. He X, Wang H, Jin T, Xu Y, Mei L, Yang J. TLR4 activation promotes bone marrow MSC proliferation and osteogenic differentiation via Wnt3a and Wnt5a signaling. PLoS One. 2016; 11:e0149876.

71. Qi C, Xiaofeng X, Xiaoguang W. Effects of toll-like receptors 3 and 4 in the osteogenesis of stem cells. Stem Cells Int. 2014; 2014:917168.

72. Zhang WQ, Huang SH, Huang X, Li JH, Ye P, Xu J, Zheng PZ, Shen HY, Huang JR. Regulation of human mesenchymal stem cell differentiation by TREM-2. Hum Immunol. 2016; 77:476–482.

73. Mo IF, Yip KH, Chan WK, Law HK, Lau YL, Chan GC. Prolonged exposure to bacterial toxins downregulated expression of toll-like receptors in mesenchymal stromal cell-derived osteoprogenitors. BMC Cell Biol. 2008; 9:52.

74. Chen X, Zhang ZY, Zhou H, Zhou GW. Characterization of mesenchymal stem cells under the stimulation of Toll-like receptor agonists. Dev Growth Differ. 2014; 56:233–244.

75. Raicevic G, Najar M, Pieters K, De BC, Meuleman N, Bron D, Toungouz M, Lagneaux L. Inflammation and Toll-like receptor ligation differentially affect the osteogenic potential of human mesenchymal stromal cells depending on their tissue origin. Tissue Eng Part A. 2012; 18:1410–1418.

76. Valtieri M, Sorrentino A. The mesenchymal stromal cell contribution to homeostasis. J Cell Physiol. 2008; 217:296–300.

77. Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010; 466:829–834.

78. Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011; 34:590–601.

79. Ziegler P, Boettcher S, Takizawa H, Manz MG, Brummendorf TH. LPS-stimulated human bone marrow stroma cells support myeloid cell development and progenitor cell maintenance. Ann Hematol. 2016; 95:173–178.

80. Iwamura C, Bouladoux N, Belkaid Y, Sher A, Jankovic D. Sensing of the microbiota by NOD1 in mesenchymal stromal cells regulates murine hematopoiesis. Blood. 2017; 129:171–176.

81. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013; 13:392–402.

82. Hwang SH, Cho HK, Park SH, Lee W, Lee HJ, Lee DC, Oh JH, Park SH, Kim TG, Sohn HJ, Kang JM, Kim SW. Toll like receptor 3 & 4 responses of human turbinate derived mesenchymal stem cells: stimulation by double stranded RNA and lipopolysaccharide. PLoS One. 2014; 9:e101558.

83. Tomchuck SL, Henkle SL, Coffelt SB, Betancourt AM. Toll-like receptor 3 and suppressor of cytokine signaling proteins regulate CXCR4 and CXCR7 expression in bone marrow-derived human multipotent stromal cells. PLoS One. 2012; 7:e39592.

84. Najar M, Raicevic G, Fayyad-Kazan H, De BC, Bron D, Toungouz M, Lagneaux L. Impact of different mesenchymal stromal cell types on T-cell activation, proliferation and migration. Int Immunopharmacol. 2013; 15:693–702.

85. Dumitru CA, Hemeda H, Jakob M, Lang S, Brandau S. Stimulation of mesenchymal stromal cells (MSCs) via TLR3 reveals a novel mechanism of autocrine priming. FASEB J. 2014; 28:3856–3866.

86. Yan H, Wu M, Yuan Y, Wang ZZ, Jiang H, Chen T. Priming of Toll-like receptor 4 pathway in mesenchymal stem cells increases expression of B cell activating factor. Biochem Biophys Res Commun. 2014; 448:212–217.

87. Levin S, Pevsner-Fischer M, Kagan S, Lifshitz H, Weinstock A, Gataulin D, Friedlander G, Zipori D. Divergent levels of LBP and TGFbeta1 in murine MSCs lead to heterogenic response to TLR and proinflammatory cytokine activation. Stem Cell Rev. 2014; 10:376–388.

88. Lei J, Wang Z, Hui D, Yu W, Zhou D, Xia W, Chen C, Zhang Q, Wang Z, Zhang Q, Xiang AP. Ligation of TLR2 and TLR4 on murine bone marrow-derived mesenchymal stem cells triggers differential effects on their immunosuppressive activity. Cell Immunol. 2011; 271:147–156.

89. Weinstock A, Pevsner-Fischer M, Porat Z, Selitrennik M, Zipori D. Cultured mesenchymal stem cells stimulate an immune response by providing immune cells with Toll-like receptor 2 ligand. Stem Cell Rev. 2015; 11:826–840.

90. Rashedi I, Gomez-Aristizabal A, Wang XH, Viswanathan S, Keating A. TLR3 or TLR4 activation enhances mesenchymal stromal cell-mediated Treg induction via notch signaling. Stem Cells. 2017; 35:265–275.

91. Wang X, Zhu Y, Xu B, Wang J, Liu X. Identification of TLR2 and TLR4induced microRNAs in human mesenchymal stem cells and their possible roles in regulating TLR signals. Mol Med Rep. 2016; 13:4969–4980.

92. Zhao X, Liu D, Gong W, Zhao G, Liu L, Yang L, Hou Y. The toll-like receptor 3 ligand, poly(I:C), improves immunosuppressive function and therapeutic effect of mesenchymal stem cells on sepsis via inhibiting MiR-143. Stem Cells. 2014; 32:521–533.

93. Sioud M, Mobergslien A, Boudabous A, Floisand Y. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol. 2010; 71:267–274.

94. Gieseke F, Kruchen A, Tzaribachev N, Bentzien F, Dominici M, Muller I. Proinflammatory stimuli induce galectin-9 in human mesenchymal stromal cells to suppress T-cell proliferation. Eur J Immunol. 2013; 43:2741–2749.

95. Cho KA, Park M, Kim YH, Ryu KH, Woo SY. Poly I:C primes the suppressive function of human palatine tonsil-derived MSCs against Th17 differentiation by increasing PD-L1 expression. Immunobiology. 2017; 222:394–398.

96. Qiu Y, Guo J, Mao R, Chao K, Chen BL, He Y, Zeng ZR, Zhang SH, Chen MH. TLR3 preconditioning enhances the therapeutic efficacy of umbilical cord mesenchymal stem cells in TNBS-induced colitis via the TLR3-Jagged-1-Notch-1 pathway. Mucosal Immunol. 2016; DOI: 10.1038/mi.2016.78.

97. Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nat Rev Immunol. 2008; 8:81–86.

98. Cassatella MA, Mosna F, Micheletti A, Lisi V, Tamassia N, Cont C, Calzetti F, Pelletier M, Pizzolo G, Krampera M. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells. 2011; 29:1001–1011.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download