1. Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011; 11:865–872.

2. Kwok R. Vaccines: the real issues in vaccine safety. Nature. 2011; 473:436–438.

3. Gutjahr A, Tiraby G, Perouzel E, Verrier B, Paul S. Triggering intracellular receptors for vaccine adjuvantation. Trends Immunol. 2016; 37:573–587.

4. Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010; 10:787–796.

5. McGhee JR, Fujihashi K. Inside the mucosal immune system. PLoS Biol. 2012; 10:e1001397.

6. Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008; 1:31–37.

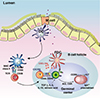
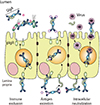
7. Corr SC, Gahan CC, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol. 2008; 52:2–12.

8. Ohno H. Intestinal M cells. J Biochem. 2016; 159:151–160.

9. Schulz O, Pabst O. Antigen sampling in the small intestine. Trends Immunol. 2013; 34:155–161.

10. Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol. 2011; 29:273–293.

11. Bemark M, Boysen P, Lycke NY. Induction of gut IgA production through T cell-dependent and T cell-independent pathways. Ann N Y Acad Sci. 2012; 1247:97–116.

12. Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012; 12:821–832.

13. Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010; 8:656–667.

14. Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012; 12:592–605.

15. Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010; 33:451–463.

16. Kim SH, Jang YS. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Exp Mol Med. 2014; 46:e85.

17. Pedersen G, Cox R. The mucosal vaccine quandary: intranasal vs. sublingual immunization against influenza. Hum Vaccin Immunother. 2012; 8:689–693.

18. Lamichhane A, Azegamia T, Kiyonoa H. The mucosal immune system for vaccine development. Vaccine. 2014; 32:6711–6723.

19. Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013; 6:666–677.

20. Clark MA, Jepson MA, Simmons NL, Hirst BH. Differential surface characteristics of M cells from mouse intestinal Peyer’s and caecal patches. Histochem J. 1994; 26:271–280.

21. Foster N, Clark MA, Jepson MA, Hirst BH. Ulex europaeus 1 lectin targets microspheres to mouse Peyer's patch M-cells in vivo. Vaccine. 1998; 16:536–541.

22. Hirabayashi J, Hashidate T, Arata Y, et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002; 1572:232–254.

23. Kim SH, Jung DI, Yang IY, et al. M cells expressing the complement C5a receptor are efficient targets for mucosal vaccine delivery. Eur J Immunol. 2011; 41:3219–3229.

24. Kim SH, Lee HY, Jang YS. Expression of the ATP-gated P2X7 receptor on M cells and its modulating role in the mucosal immune environment. Immune Netw. 2015; 15:44–49.

25. Kim SH, Yang IY, Kim J, Lee KY, Jang YS. Antimicrobial peptide LL-37 promotes antigen-specific immune responses in mice by enhancing Th17-skewed mucosal and systemic immunities. Eur J Immunol. 2015; 45:1402–1413.

26. Clark MA, Hirst BH, Jepson MA. M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect Immun. 1998; 66:1237–1243.

27. Hase K, Kawano K, Nochi T, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009; 462:226–230.

28. Keely S, Glover LE, Weissmueller T, et al. Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. Mol Biol Cell. 2010; 21:538–546.

29. Kunisawa J, Kurashima Y, Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv Drug Deliv Rev. 2012; 64:523–530.

30. Kyd JM, Cripps AW. Functional differences between M cells and enterocytes in sampling luminal antigens. Vaccine. 2008; 26:6221–6224.
31. Lo DD, Ling J, Eckelhoefer AH. M cell targeting by a Claudin 4 targeting peptide can enhance mucosal IgA responses. BMC Biotechnol. 2012; 12:7.
32. Nakato G, Hase K, Suzuki M, et al. Cutting Edge: Brucella abortus exploits a cellular prion protein on intestinal M cells as an invasive receptor. J Immunol. 2012; 189:1540–1544.

33. Nochi T, Yuki Y, Matsumura A, et al. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J Exp Med. 2007; 204:2789–2796.

34. Osanai A, Sashinami H, Asano K, Li SJ, Hu DL, Nakane A. Mouse peptidoglycan recognition protein PGLYRP-1 plays a role in the host innate immune response against Listeria monocytogenes infection. Infect Immun. 2011; 79:858–866.

35. Rand JH, Wu XX, Lin EY, Griffel A, Gialanella P, McKitrick JC. Annexin A5 binds to lipopolysaccharide and reduces its endotoxin activity. MBio. 2012; 3:pii: e00292-11.

36. Sato S, Kaneto S, Shibata N, et al. Transcription factor Spi-B-dependent and -independent pathways for the development of Peyer's patch M cells. Mucosal Immunol. 2013; 6:838–846.

37. Shafique M, Wilschut J, de Haan A. Induction of mucosal and systemic immunity against respiratory syncytial virus by inactivated virus supplemented with TLR9 and NOD2 ligands. Vaccine. 2012; 30:597–606.

38. Tyrer P, Foxwell AR, Cripps AW, Apicella MA, Kyd JM. Microbial pattern recognition receptors mediate M-cell uptake of a gram-negative bacterium. Infect Immun. 2006; 74:625–631.

39. Wolf JL, Kauffman RS, Finberg R, Dambrauskas R, Fields BN, Trier JS. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology. 1983; 85:291–300.

40. Kim SH, Seo KW, Kim J, Lee KY, Jang YS. The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol. 2010; 185:5787–5795.

41. Agren LC, Ekman L, Lowenadler B, Lycke NY. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J Immunol. 1997; 158:3936–3946.

42. Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010; 33:479–491.

43. Christensen D, Agger EM, Andreasen LV, Kirby D, Andersen P, Perrie Y. Liposome-based cationic adjuvant formulations (CAF): past, present, and future. J Liposome Res. 2009; 19:2–11.

44. Eliasson DG, Helgeby A, Schon K, et al. A novel non-toxic combined CTA1-DD and ISCOMS adjuvant vector for effective mucosal immunization against influenza virus. Vaccine. 2011; 29:3951–3961.

45. Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Semin Immunol. 2009; 21:185–193.

46. Thompson AL, Johnson BT, Sempowski GD, et al. Maximal adjuvant activity of nasally delivered IL-1alpha requires adjuvant-responsive CD11c(+) cells and does not correlate with adjuvant-induced in vivo cytokine production. J Immunol. 2012; 188:2834–2846.

47. Uematsu S, Fujimoto K, Jang MH, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008; 9:769–776.

48. van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. Chitosan for mucosal vaccination. Adv Drug Deliv Rev. 2001; 52:139–144.

49. Winstone N, Wilson AJ, Morrow G, et al. Enhanced control of pathogenic Simian immunodeficiency virus SIVmac239 replication in macaques immunized with an interleukin-12 plasmid and a DNA prime-viral vector boost vaccine regimen. J Virol. 2011; 85:9578–9587.

50. Agren L, Sverremark E, Ekman L, et al. The ADP-ribosylating CTA1-DD adjuvant enhances T cell-dependent and independent responses by direct action on B cells involving anti-apoptotic Bcl-2- and germinal center-promoting effects. J Immunol. 2000; 164:6276–6286.

51. Mastelic Gavillet B, Eberhardt CS, Auderset F, et al. MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. J Immunol. 2015; 194:4836–4845.










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download