Abstract
Recently, patients with shoulder pain have increased rapidly. Of all shoulder disorders, rotator cuff tears (RCTs) are most prevalent in the middle-aged and older adults, which is the primary reason for shoulder surgery in the population. Some authors have reported that up to 30% of total RCTs can be classified as irreparable due to the massive tear size and severe muscle atrophy. In this review article, we provide an overview of treatment methods for irreparable massive RCTs and discuss proper surgical strategies for RCTs that require operative management.
The outcome of rotator cuff tear (RCT) repair has been improved by recent advances in surgical techniques and instruments including arthroscopy. Nevertheless, the reported treatment failure rate is approximately 40% for massive RCTs encompassing greater than 5 cm tears or tears involving two or more tendons.1) Some authors23) have reported that up to 30% of total RCTs can be classified as irreparable due to the massive tear size and severe muscle atrophy. With the rapid growth of the aging population, we have witnessed an accelerating increase in the number of patients complaining of shoulder pain. People's interest in shoulder disorders is also growing not only to be able to perform daily living activities and occupational activities but also to engage in sports. Of all shoulder disorders, RCTs are most prevalent in the middle-aged and older adults, which is the primary reason for shoulder surgery in the population. Therefore, it is imperative for surgeons to improve treatment strategies for RCTs to obtain better postoperative outcomes. Unfortunately, it is difficult to accurately predict the reparability of an RCT based on preoperative clinical presentations and magnetic resonance imaging (MRI) findings. A successful repair does not necessarily translate into functional improvement of the shoulder. In addition, in spite of the growing popularity of arthroscopic repair, studies showing the long-term promise of reverse total shoulder arthroplasty (RTSA) have ensued. When all these factors are taken into consideration, it is evident that surgical treatment of RCTs deemed irreparable requires an extremely challenging and highly important decision-making process. In other words, the surgeon has to determine the optimal surgical approach based on comprehensive assessment of the patient's age, activity demands, tear pattern, integrity of the remaining rotator cuff, probability of treatment success, and familiarity with the surgical technique. In this review article, we provide an overview of treatment methods for irreparable massive RCTs and discuss proper surgical strategies for RCTs that require operative management.
Levy et al.4) reported that an anterior deltoid rehabilitation program (Fig. 1) was effective in improving range of motion and Constant score from a mean of 26 before treatment to a mean of 60 after treatment in elderly patients diagnosed with irreparable RCTs. In a study by Yian et al.,5) the success rate of anterior deltoid rehabilitation in 30 elderly patients with irreparable RCTs was 40%. Gialanella and Bertolinelli6) suggested that intra-articular steroid injections could be effective for pain relief in RCT patients. However, repeated steroid injections weakened rotator cuffs and negatively affected bone quality in a rat model in a study by Maman et al.7) Therefore, the limitations of steroid injection as a primary treatment have been widely recognized; it can be an effective adjuvant when combined with other conservative treatment.
The above-mentioned studies have demonstrated that conservative treatment is useful for improving range of motion (shoulder elevation, internal rotation, and external rotation), strengthening muscle power, and reducing pain in elderly patients with low activity levels and patients unfit for surgery due to other comorbidities that preclude surgery. It can be considered the best primary treatment option for symptoms developing or worsening after trauma or pain responding well to nonoperative treatment without interfering with normal daily life. However, considering the natural history of RCTs, for young patients, it is advised to perform regular radiological follow-up to monitor changes in the rotator cuff.
Arthroscopic debridement with subacromial decompression was first introduced by Rockwood et al.8) for the treatment of irreparable RCTs in 1995. In the study, of the 50 patients with irreparable RCTs, 83% had pain reduction and improved range of motion at a mean follow-up of 6.5 years after the procedure. Specifically, the mean active forward flexion of the shoulder increased from 105° preoperatively to 140° postoperatively. In a study by Kempf et al.,9) 73% of the 210 shoulders with RCTs obtained satisfying results at a mean of 26.6 months after arthroscopic debridement and acromioplasty. Veado and Rodrigues10) also observed improvement in the University of California, Los Angeles (UCLA) score and pain in 22 irreparable RCT patients who underwent arthroscopic debridement although there was no improvement in muscle strength.
Arthroscopic debridement with subacromial decompression can be performed in patients who are able to do active motion of the shoulder with consisting pain after conservative treatment. In addition, elderly patients, patients who have various systemic diseases, and patients who are in a rehabilitation program could also be indications. For massive RCT patients enduring recurrent relapse and remission of symptoms with conservative treatment, a synergetic effect can be expected from arthroscopic debridement with subacromial decompression. Although the retracted rotator cuff muscles cannot be repaired by the procedure in such patients, excision of inflammatory tissue and anatomical structures responsible for impingement will contribute to functional improvement and pain relief after conservative therapy.
Tuberoplasty was first introduced in open surgery by Fenlin et al.11) in 2002. The concept is to create a smooth, congruent acromiohumeral articulation by removing the exostoses on the humerus and reshaping the greater tuberosity. During the procedure, the coracoacromial ligament is preserved and acromioplasty is not performed. In the 2002 study involving 21 patients for a mean of 27 months, the UCLA score improved from a mean of 9.3 preoperatively to a mean of 27.7 postoperatively, and the results were satisfactory in 95% of the patients (12, excellent; 6, good; and 1, fair). Postoperatively, 68% of the patients reported they were pain-free, but residual weakness in external rotation was observed in all patients. In 2004, Scheibel et al.12) presented an arthroscopic approach to tuberoplasty, which was termed reversed arthroscopic subacromial decompression. The procedure requires an arthroscopic debridement of the subacromial space and glenohumeral joint prior to tuberoplasty. Twenty-three patients in the study were followed for a mean of 40 months after surgery: the Constant score increased, and significant improvement was obtained in terms of pain and range of motion.
Verhelst et al.13) followed up 34 shoulders with RCTs for a mean of 38 months after reversed arthroscopic subacromial decompression. The modified Constant score increased from a mean of 34.9% to 84.0%, pain was reduced, and range of motion was increased. In the meantime, however, the mean acromiohumeral distance (AHD) decreased by 2.58 mm and the severity of glenohumeral osteoarthritis increased. Overall, they concluded that the procedure could be beneficial for elderly patients with irreparable RCTs. Lee et al.14) reported the results of arthroscopic tuberoplasty in 32 patients with irreparable RCTs. In 26 patients, acromioplasty was performed concomitant to arthroscopic tuberoplasty, compared to the six patients who only had tuberoplasty. The mean Constant score increased from 47.6 preoperatively to 70.4 postoperatively; the UCLA score increased from a mean of 15.4 preoperatively to a mean of 27.1 postoperatively. The mean active range of forward flexion improved from 115.9° preoperatively to 142.7° postoperatively. In a clinical follow-up, the patients who had acromioplasty as well as arthroscopic tuberoplasty obtained superior pain relief and improvement in active forward flexion. They suggested that preservation of the acromiohumeral interval (AI) and continuity in the inferior scapulohumeral line are prognostic factors for good clinical outcomes. Park et al.15) also demonstrated the efficacy of tuberoplasty in 16 patients with an approximately 8 years of follow-up (mean, 98 months). The visual analogue scale (VAS) score for pain decreased from a mean of 6.9 preoperatively to a mean of 2.3 postoperatively. The mean UCLA score increased from 10.3 preoperatively to 27.2 postoperatively. The mean Constant score improved from 37.9 preoperatively to 59.2 postoperatively. The AI decreased from 5 mm preoperatively to 4 mm at the last follow-up. In the long-term follow-up, the patients had excellent functional outcome, but more substantial superior migration of the humeral head occurred.
We think that tuberoplasty can be concomitantly performed with acromioplasty if deemed necessary. However, it should be noted that although it is effective for alleviating pain and improving function scores and range of motion, tuberoplasty alone can result in reduced AI and increased superior migration of the humeral head in the long term as described in previous studies. Therefore, it is of more importance to reestablish dynamic force couples with partial rotator cuff repair to maintain a proper AI. In a previous study, we assessed 173 patients who underwent arthroscopic repair of massive RCTs for a mean follow-up of 31 months.1) In the study, we found poor functional outcome was associated with an AHD of less than 4.3 mm. Considering that the postoperative AHD is the only determinant factor of postoperative shoulder function, it is of vital importance to maintain AHD to be 4.3 mm or more with all available surgical methods.
In 1993, Burkhart et al.1617) first introduced the concept of suspension bridge in RCTs, which developed into functional cuff tears and formed the theoretical basis of partial repair of the rotator cuff. They reported good outcomes with repair of the margins of the torn rotator cuff designed to restore the force couples and facilitate force transmission. The mean active elevation improved from 59.6° preoperatively to 150.4° postoperatively. Strength assessed on a scale of 0–5 improved from an average of 2.1 preoperatively to an average of 4.4 postoperatively. The UCLA score improved from a mean of 9.8 preoperatively to a mean of 27.6 postoperatively. In a study by Kim et al.,18) 27 patients who underwent partial repair of irreparable RCTs obtained satisfying outcomes. The mean preoperative tear size was 42.1 mm and the mean residual defect size was 12.0 mm. The Simple Shoulder Test (SST) improved from a mean of 5.1 preoperatively to a mean of 8.8 postoperatively. The Constant score increased from a mean of 43.6 preoperatively to a mean of 74.1 postoperatively, and the UCLA score improved from a mean of 10.5 preoperatively to a mean of 25.9 postoperatively. Galasso et al.19) also demonstrated similar results of partial repair in 95 shoulders with a mean follow-up of 7 years. The Constant score improved from a mean of 39.1 preoperatively to a mean of 76.3 postoperatively and the mean postoperative SST was 9.1.
Iagulli et al.20) reported that the outcomes of partial repair of massive RCTs were comparable to those of complete repair. At a mean of 24 months of follow-up, the UCLA score in the complete repair group (52 patients) was 29.64, which was not significantly different from 29.49 in the partial repair group (45 patients). Berth et al.21) compared the outcomes of arthroscopic debridement and partial repair in patients with massive RCTs. The two surgical methods were comparably effective in pain relief, but partial repair was superior in terms of functional outcome. In addition, the 24-month postoperative ultrasonography revealed 52% structural failure of the partially repaired rotator cuffs.
Not all massive RCTs are surgically irreparable. Therefore, best efforts should be made to reattach the anterior aspect of the infraspinatus and superior aspect of the subscapularis to the tubercles of the humerus with minimal tension for restoration of normal biomechanics of the shoulder, in which force coupling should return, eventually improving pain and functional outcomes. Compared to the arthroscopic debridement alone, partial repair performed to the fullest possible extent should facilitate superior functional improvement. In our previous study, we compared the impact of partial repair and complete repair on glenohumeral biomechanics in eight cadaveric shoulders with massive RCTs: posterior fixation was important in restoring abnormal glenohumeral kinematics.22) Therefore, we recommend partial repair of the rotator cuff as much as possible, even in the case of massive tears, with medialization or rotator interval release for minimal tension. If necessary, it may be followed by additional procedures such as balloon spacer placement and superior capsular reconstruction (SCR). There is no contraindication limited to partial repair. Similar to complete repair, if osteoarthritic change is present in the glenohumeral joint, it is reasonable to consider arthroplasty rather than repair.
There are a variety of surgical techniques for reattachment to the anatomical footprint of the margins of medially retracted rotator cuff tears that cannot be mobilized to the humeral tuberosity.
Interval slide was first introduced in open surgery by Bigliani et al.23) in 1992, and Tauro24) popularized arthroscopic interval slide, which involves release of the torn, retracted supraspinatus tendon from the rotator interval for improved mobility.25) Lo and Burkhart26) defined the anterior interval slide as release from the rotator interval and the posterior interval slide as release of the interval between the supraspinatus and the infraspinatus tendons. They reported excellent outcomes of repair of severely contracted, massive RCTs with an interval slide technique for increased mobility of the supraspinatus tendon in nine shoulders. At a mean follow-up of 17.9 months, the mean UCLA score increased from 10.0 preoperatively to 28.3 postoperatively. Active forward flexion and active external rotation improved from a mean of 108° preoperatively to a mean of 146.1° postoperatively and from a mean of 24.4° preoperatively to a mean of 35.0° postoperatively, respectively.
However, there is some concern that interval slide techniques can cause devascularization of the supraspinatus. Kim et al.27) compared the 2-year follow-up results of complete repair by an interval slide with those of partial repair following margin convergence. The SST, American Shoulder and Elbow Surgeons (ASES) score, and UCLA score improved in both groups without showing and significance intergroup difference. In addition, 6-month MRI revealed a retear in 91% of the complete repair group. Therefore, they concluded that complete repair using an interval slide technique has no advantages over partial repair in terms of treatment outcome. Berdusco et al.28) reported a 55% retear rate using an interval slide technique in patients with massive, contracted, and immobile rotator cuff tears, followed by MRI at an average of 25.2 months after surgery.
Based on our clinical experiences, we know that interval slide does not allow for sufficient mobilization and the nonanatomical reattachment in the posterior interval slide may increase the risk of devascularization of the supraspinatus tendon. It is our understanding that mobility of the retracted rotator cuff can be sufficiently improved with a release of the coracohumeral ligament from the coracoid under surface (Fig. 2) instead of a release from the rotator interval. For these reasons, we do not use the traditional interval slide techniques at our institution.
Burkhart et al.29) and Burkhart30) suggested margin convergence (Fig. 3) as a method of reducing strain and increasing the fixations strength. In a 2-year follow-up study by Shindle et al.,31) the margin convergence technique performed in U-shaped RCTs resulted in improvement in the ASES score (from a mean of 50.0 preoperatively to a mean of 83.3 postoperatively), active forward elevation (from a mean of 156.2° preoperatively to a mean of 168° postoperatively), and active external rotation (from a mean of 54.4° preoperatively to a mean of 57.1° postoperatively). However, the 2-year follow-up ultrasonography revealed that only 46.2% of the RCTs were healed. In a biomechanical study of margin convergence, Mazzocca et al.32) reported that the technique decreased rotator cuff strain at all degrees of rotation and gap size in 20 cadaveric shoulders. In our previous comparison study of partial repair and complete repair, the shoulders were biomechanically less stable after partial repair in spite of good clinical outcomes. Furthermore, we emphasized the importance of anterior margin convergence in decreasing gap formation and restoring range of motion and posterior infraspinatus repair in restoring abnormal humeral head apex kinematics.22) However, we are doubtful about the efficacy of margin convergence for severely degenerated poorly vascularized rotator cuffs. If necessary, margin convergence performed at the muscle-tendon junction may be more conducive to biological healing of the rotator cuff.
Medialization can be considered when reattachment of a torn tendon to the anatomical footprint is not feasible in irreparable RCTs. In a biomechanical study by Liu et al.,33) 3 mm and 10 mm medial advancement of the tendon had a negligible impact on the moment arm during elevation, whereas 17 mm medial advancement negatively affected biomechanics of the shoulder by significantly reducing the moment arm in 10 fresh-frozen cadaveric shoulders. Yamamoto et al.34) reported that ≥ 10 mm medialization in 10 cadaveric shoulders resulted in significant limitation in range of motion. Kim et al.35) reported that medialized repair in 35 RCT patients improved clinical outcomes: the mean VAS score, from 6 preoperatively to 2 postoperatively; the mean active forward elevation, from 134° preoperatively to 150° postoperatively; the mean active external rotation, from 47° preoperatively to 55° postoperatively; the mean Constant score, from 53.5 preoperatively to 79 postoperatively; the mean ASES score, from 51 preoperatively to 82 postoperatively; and the mean UCLA score, from 14 preoperatively to 28 postoperatively. The retear rate at the last follow-up was 17% in the study. Therefore, we suggest that < 10 mm medialization can be a viable option for irreparable RCTs without significant negative consequences on biomechanics and limitation on the range of motion of the shoulders.
Considering the postoperative biomechanical stability demonstrated in previous clinical and biomechanical studies, complete repair should be attempted whenever possible. The above-described techniques including interval slide, margin convergence, and medialization can aid in reducing tension on the tendon, minimizing gap formation and restoring normal kinematics of the humeral head for complete repair.
Graft augmentation (Fig. 4) has demonstrated mechanical and biological advantages. Xenograft, synthetic materials, and allograft have been used to augment rotator cuff repair. The currently available xenograft materials for tendon augmentation include porcine dermal collagen and small intestine submucosa. However, xenograft has not be extensively researched and it has been associated with unfavorable outcomes. Soler et al.36) used porcine dermal collagen implants (Permacol) as augmentation in the repair of four RCTs, but graft failure occurred in all patients. Sclamberg et al.37) also confirmed retears at 6-month follow-up MRI in 10 of 11 patients after repair reinforced with porcine small intestinal submucosa. In a study by Iannotti et al.,38) porcine small intestine submucosa augmentation of rotator cuff repair was not helpful in improving tendon healing and clinical outcomes.
Synthetic augmentation is advantageous for high mechanical elasticity and the use of nonabsorbable devices improve mechanical stability. Encalada-Diaz et al.39) performed rotator cuff repair augmented with a polycarbonate polyurethane patch in patients with full-thickness RCTs. At 1-year follow-up, MRI-confirmed healing was obtained in 90% without any complication. In a study by Ciampi et al.,40) the 12-year follow-up ultrasonography showed a retear rate of 41% in the control group compared to 17% in the synthetic patch augmentation group. At the 3-year follow-up, the latter group also obtained higher UCLA scores and greater abduction strength than the control group. However, biologically inactive synthetic implants do not provide regenerative stimuli that support the healing process.
The use of a freeze-dried graft for repair of massive RCTs was first attempted by Neviaser et al.41) in 1978, which resulted in excellent outcomes in 14 of 16 patients. By contrast, Nasca42) reported in 1988 that functional improvement was obtained with the use of freeze-dried allografts in only two of seven patients. In a study by Moore et al.,43) the outcomes of allograft reconstruction of RCTs were similar to those of debridement and subacromial decompression without grafting. On the other hand, in a prospective study reported by Barber et al.44) in 2012, the healing rate was significantly higher in the group with GraftJacket acellular human dermal matrix (Wright Medical Technology, Arlington, TN, USA) augmentation than in the group without augmentation (85% vs. 40%).
In our previous publication, we compared the use of autogenic biceps graft (24 patients) and an allogenic dermal patch (eight patients) for irreparable massive RCTs. In the autogenic biceps graft group, there was improvement in the shoulder function score, pain, and range of motion, and the 1-year follow-up retear rate was 54.2%. By contrast, in the allogenic dermal patch graft group, there was no notable improvement in function score and range of motion, and only pain was significantly reduced. In addition, the 1-year follow-up retear rate was 75% in them.45) Some studies have suggested methods to overcome failure after massive RCT repair. Yoon et al.46) performed patch augmentation and bone marrow stimulation in 21 patients with massive RCTs and compared the results with those of conventional repair performed in 54 patients. The 1-year follow-up MRI showed a retear rate of 19% in the former group and 46.3% in the conventional repair group. In particular, the medial-row failure (type 2 retear) rate was 0% in the patch augmentation and bone marrow stimulation group, whereas the rate was 72.0% among the retear cases (18/25) after conventional repair. Graft augmentation could decrease high tension applied to the torn tendon while pulling the torn tendon edge to the lateral footprint. Especially, the reduced tension at the medial-row repaired site may have decreased the rate of type 2 retear. In the past, we investigated whether allogenic dermal patches can serve as a cytokine carrier using a rabbit model.47) Eighty white rabbits with supraspinatus tendon tears were allocated into four different treatment groups consisting of 20 each: repair only group, repair + patch augmentation group, repair + platelet-rich plasma injection group, and repair + patch augmentation + platelet-rich plasma injection group. The vascularity and cellularity were higher in the groups treated with platelet-rich plasma than those without. The collagen fiber continuity and orientation were also better in the platelet-rich plasma treatment groups. However, there was no notable difference in tendon to bone healing between the patch augmented groups and nonaugmented groups. Therefore, we could not confirm the function of allogenic dermal tissue as a cytokine carrier in the study.
On the basis of conflicting outcomes documented in previous studies, the efficacy of allograft has yet to be elucidated in further research. Since differences in the composition of study population and the purpose of grafting (bridging or augmentation) affect the resultant healing rates,45) it is of utmost importance to ensure proper patient selection. Compared to concomitant multiple channeling and augmentation,46) the outcomes of platelet-rich plasma injection plus graft augmentation reported in recent studies have been less than satisfactory.47) Therefore, future research should explore the optimal treatment combination with biologic augmentation to improve healing after massive RCT repair.
Biceps augmentation (Fig. 5) was first described by Neviaser48) in 1971. Rhee et al.49) reported a mean of 31-month follow-up results of biceps augmentation for massive RCT repair. The procedure was performed arthroscopically in 16 cases and without arthroscopy in 15 cases. Regardless of the type of procedure, the function scores were improved without any significant intergroup difference. In the arthroscopic augmentation group, complete healing was confirmed with MRI in 64%. In a study by Cho et al.,50) rotator cuff repair performed with biceps augmentation (37 cases) and without augmentation (31 cases) did not show difference in terms of improvement in the UCLA score. However, significant improvement in forward flexion, external rotation, and internal rotation strength was observed only in the augmentation group. The rotator cuff healing rate was 58.3% in the augmentation group, whereas the rate was 26.3% without augmentation. Ji et al.51) followed 35 patients for a mean of 24 months after massive RCT repair augmented with the long head of the biceps tendon: functions scores improved and complete healing was obtained in 63%. Therefore, if biceps tendons remain intact in massive RCTs, augmentation of the defect in the rotator cuff during repair can be expected to prevent superior migration of the humeral head and provide clinical improvement although a retear may occur in 35%–40%.
Tendon transfer is one of the surgical treatment options for irreparable massive RCTs among relatively young patients without glenohumeral osteoarthritis. The biomechanical rationale for tendon transfer is restoration of force couples of the glenohumeral joint and normal kinematics of the shoulder.52) The most frequently employed and studied tendon transfer techniques are latissimus dorsi transfer and pectoralis major transfer.
Latissimus dorsi transfer can be used to replicate posterior force couple of the infraspinatus and the teres minor muscle. During the procedure, the insertion of the latissimus dorsi on the lesser tuberosity is transferred to the greater tuberosity of the humerus, which converts this internal rotator of the shoulder to an external rotator. In the presence of a posterior RCT, the relatively high muscle strength of the anterior rotator cuff results in increased internal rotation and a significant external rotation deficit. Under such circumstances, transferring an internal rotator into an external rotator was suggested as a method to maintain force couples and restore normal kinematics in irreparable RCTs. However, it should be noted that after a latissimus dorsi transfer, the line of pull is more vertical than the force vector of the posterior rotator cuff muscles. In a biomechanical research, Oh et al.52) showed that latissimus dorsi transfer is advantageous for restoring internal/external rotational range of motion and balance of the glenohumeral joint. At the same time, they pointed out that limited excursion at 60° of abduction may cause an overcompensation phenomenon and increased contact pressure, further deteriorating normal biomechanics of the shoulder. In addition, abnormal kinematics of the humeral head can result in persisting pain and glenohumeral osteoarthritis. In a study by Aoki et al.,53) active forward flexion improved after a mean of 35 months of latissimus dorsi transfer, but osteoarthritic changes were observed in 41%. Gerber et al.54) also reported that range of motion, muscle strength, and function scores improved after latissimus dorsi transfer (n = 69), but osteoarthritic changes progressed in 30%. In addition, they found that insufficiency of the subscapularis was associated with unfavorable postoperative outcomes. In a biomechanical study by Werner et al.,55) the relationship between the presence of a subscapularis tendon tear and inferior results of latissimus dorsi transfer was demonstrated in a cadaveric model.
Recently, arthroscopically-assisted latissimus dorsi transfer has been shown to provide favorable clinical outcomes.56) The long-term clinical outcome of the procedure was also promising in a recent study: successful functional improvement was observed at a mean of 9.3 years with only 10% of clinical failure.57) As Henseler et al.58) suggested, synergistic muscle activity of the latissimus dorsi during arm abduction and external rotation confirmed at 1-year electromyography may indicate that restoration of active external rotation is achieved by active muscle contraction of the transferred latissimus dorsi rather than passive tenodesis effect.
It has yet to be elucidated whether favorable clinical outcome after latissimus dorsi transfer is due to active muscle contraction rather than passive tenodesis effect. However, based on the 10-year follow-up results and anatomical biomechanical research, we believe latissimus dorsi transfer can be used with success to improve the outcome of RCT repair when performed exclusively in relatively young patients without osteoarthritis or subscapularis insufficiency and with sufficient latissimus dorsi excursion. Since the procedure demands a long rehabilitation process, it is difficult to accurately predict the extent of recovery.
Pectoralis major transfer is used for anterosuperior RCTs. It was first described by Wirth and Rockwood in 1997 in patients with irreparable RCTs.59) Resch et al.59) reported that pain relief and improvement in the Constant score were observed at a mean of 28 months after the procedure in 12 patients. In a study by Gavriilidis et al.,60) in spite of the improved pain and Constant score, there was no increase in the range of motion in 15 patients at a mean of 37 months. Thrirteen of the total patients were available for MRI during the follow-up: the transferred pectoralis major muscle was intact in 70% of them and ruptured in 15%. Jost et al.61) performed 30 pectoralis major transfers and followed them for a mean of 32 months. They reported improved pain, range of motion, and Constant score but suggested a subscapularis tear combined with a supraspinatus tear was associated with unfavorable results.
Pectoralis major transfer can be a viable option for relatively young patients with isolated irreparable subscapularis tears without arthritis. However, it is a technically demanding procedure, and care should be taken to avoid injury to the musculocutaneous nerve.
The superior capsule of the glenohumeral joint is located on the inferior surface of the supraspinatus and infraspinatus tendons and contributes to superior stability of the joint in conjunction with the rotator cuff.62) SCR (Fig. 6) involves acromioplasty for prevention of graft abrasion and a repair of the infraspinatus and the subscapularis as much as possible. The harvested tensor fascia lata autograft, double or triple the size of the rotator cuff defect, is folded to have a thickness of 6–8 mm and inserted into the subacromial space. The graft is attached medially to the superior aspect of the glenoid tubercle using two suture anchors and laterally to the greater tuberosity using one suture anchor. Then, the graft is sutured to the infraspinatus posteriorly and the subscapularis anteriorly to restore force coupling of the joint.63)
Mihata et al.62) reported that interposition patch graft where the graft is sutured to the torn tendon partially restored superior stability, whereas SCR where the graft is attached to the superior glenoid tubercle completely restored superior stability of the shoulder joint in cadaveric shoulders with massive RCTs. In a clinical study, they also assessed the outcomes of 24 SCRs using tendor fascia lata for irreparable RCTs with a mean follow-up of 34 months: active elevation, external rotation, and ASES scores improved, and the mean AI increased from 4.6 mm preoperatively to 8.7 mm postoperatively. In a follow-up MRI, graft failure was not observed in 83.3% of the patients, and there was no case of progression of osteoarthritis or rotator cuff muscle atrophy.63) Denard et al.64) followed up 59 patients who underwent SCR with dermal allograft for a minimum of 1 year. Forward flexion, external rotation, and the ASES score improved. The AI improved from 6.6 mm preoperatively to 7.6 mm at 2 weeks postoperatively but decreased to 6.7 mm at the last follow-up. The treatment outcome was considered successful in approximately 70% of the patients.
SCR is a relatively new surgical technique that is gaining popularity for biomechanical efficacy and excellent early clinical results. It can be a reasonable treatment option for relatively young patients with irreparable RCTs unless severe osteoarthritis is present. The joint-preserving procedure improves biomechanical stability of the shoulder, but it is a technically challenging procedure that necessitates prolonged surgical time, which increases the risk of complications such as infection. The using material and effect of SCR have not yet been established. There is no long-term data comparing allograft and autograft in SCR. In addition, it is controversial whether the loading of the cuff is reduced by the SCR or the space occupying effect of the graft itself. In addition, clinical outcomes have been reported by only a few authors; thus, long-term follow-ups and other clinical studies should be conducted.
Another recent technique that is growing in popularity is biodegradable subacromial spacer insertion (InSpace system, Orthospace, Caesarea, Israel) (Fig. 7). During the procedure, a balloon-shaped spacer made of a copolymer is inserted through a lateral portal and inflated with saline to fit into the subacromial space. The device is designed to widen the acromial space and contribute to maintenance of force coupling. It acts as a subacromial spacer for 6 to 12 months and then fully degrades. The implanted balloon allows for frictionless gliding of the humeral head in the subacromial space and reduces pain, ultimately restoring the biomechanics of the glenohumeral joint.6566)
Senekovic et al.65) prospectively evaluated 20 patients with massive RTCs for a minimum of 5 years after biodegradable subacromial spacer insertion. Functional improvement was sustained during the follow-up and 84.6% of the patients were satisfied with the outcome. Deranlot et al.67) retrospectively reviewed 39 patients with irreparable massive RCTs. At a minimum of 1 year, range of motion and function scores improved. However, the patients did not undergo acromioplasty and the AI at the last follow-up decreased 2.1 mm from the preoperative value. Subacromial decompression before spacer insertion was thought unnecessary in a study by Gervasi et al.;68) however, most studies have demonstrated that it should be sufficiently performed to eliminate the source of pain, prevent damage to the device by a bone spur, and determine the appropriate balloon size for insertion.697071) Some studies suggested subacromial spacer insertion cannot be an option in patients with subscapularis tears due to the risk of anterior migration of the balloon.6567) Other studies showed that subscapularis reconstruction performed prior to subacromial spacer insertion for restoration of force coupling resulted in satisfactory outcomes.69) Regarding complications, excluding synovitis that occurred in two out of 24 patients in a study by Senekovic et al.,65) no specific complications have been reported.6970)
Studies that compare subacromial spacer insertion with other surgical techniques are rare. We also have a clinical study underway that would provide information on the efficacy of the technique, but in the meantime it is imperative to establish appropriate indications and contraindications. For now, it seems reasonable to take the following factors into consideration for successful outcome of the procedure: (1) it should be performed in patients without fixed humeral head elevation in stress X-ray or chronic pseudoparalysis; (2) sufficient subacromial decompression including acromioplasty should precede the procedure considering the subacromial spur can be a source of pain or cause damage to the device; (3) partial repair of repairable rotator cuffs, especially the subscapularis and the infraspinatus tendons, should be performed: (4) the size of the balloon should be relatively large to maintain the position after insertion; (5) considering deflation over time, the balloon should be at maximum inflation volume initially. In conclusion, subacromial spacer insertion can be a promising adjuvant procedure to partial RCT repair. It is a relatively simple technique aimed at maintaining or increasing the AI to restore force couples in the rotator cuff for the purpose of improving clinical outcomes.
Arthroplasty is commonly performed for the treatment of irreparable RCTs. It can be considered a primary treatment option for massive RCTs in the presence of advanced osteoarthritis. Arthroplasty options for irreparable RCTs include hemiarthroplasty and RTSA. Factors that should be considered when determining the treatment strategy include patient's age, level of activity, superior migration of the humeral head, extent of damage and status of the RCT, and osteoarthritis.
Hemiarthroplasty can be a viable option in patients with ≥ 90° active forward elevation when pseudoparalysis is not present. It can be done in patients who have consistent pain associated with glenohumeral osteoarthritis after conservative treatment with sparing of the glenoid articular surface, and patients who have all types of glenohumeral arthritis with inadequate glenoid bone stock are also indicated for the procedure. Goldberg et al.72) reported that 76% of the 31 patients (34 shoulders) who underwent hemiarthroplasty for the treatment of RCT arthropathy obtained excellent clinical outcomes and improvement in range of motion at a mean of 3.7 years after surgery. The improvement was more noticeable in the long-term in patients who had active forward elevation of ≥ 90° before surgery. Zuckerman et al.73) also observed pain relief and improvement in forward elevation and external rotation in 87% of 15 hemiarthroplasties performed in elderly patients with advanced RCT arthropathy for a mean follow-up of 28 months. Visotsky et al.74) reported improvement in 60 patients who underwent hemiarthroplasty for the treatment of RCT arthropathy with a mean follow-up of 2 years: active forward flexion, from 56° preoperatively to 116° postoperatively; and active external rotation, from 8° preoperatively to 30° postoperatively. In a study by Sanchez-Sotelo et al.,75) approximately 67% of 30 patients (33 shoulders) who underwent hemiarthroplasty for RCTs showed pain relief and increased range of motion during a mean follow-up of 5 years. However, anterosuperior instability occurred in seven cases that had a history of iatrogenic injury to the coracoacromial arch associated with subacromial decompression. Hemiarthroplasty may also be effective in improving pain and range of motion in patients with irreparable RCTs accompanied by glenohumeral joint arthritis. However, it may result in bone loss and instability of the humeral head, which is significantly affected by the integrity of the coracoacromial arch. Labral degeneration may also have a significant impact on clinical outcome of hemiarthroplasty. In addition, the procedure is not considered beneficial for patients with chronic pseudoparalysis.
Since Paul Grammont introduced the biomechanical concept of medialization of the center of rotation and inferior translation of the humeral head in 1985, RTSA has continuously evolved into an alternative to total shoulder arthroplasty for various shoulder disorders that cannot be managed with the traditional arthroscopic procedure.76) In the procedure, medialization of the glenohumeral center of rotation increases the moment arm of the deltoid muscle and inferior translation of the rotation center lengthens the lever arm to optimize the efficiency of the deltoid muscle. RCTs with superior migration of the humeral head in the presence of osteoarthritis are standard indications for RTSA for optimal clinical outcome. In particular, it can be a solution for painful pseudoparalysis. Sirveaux et al.77) assessed the efficacy of RTSA in 80 shoulders with massive rupture of the cuff with a mean follow-up of 44 months: the Constant score improved from 22.6 preoperatively to 65.6 postoperatively; active forward elevation improved from 73° preoperatively to 138° postoperatively; and 96% of the patients had pain relief. Frankle et al.78) followed up 60 patients who had RTSA for the treatment of rotator cuff deficiency for a mean of 2 years: the mean active forward elevation increased from 55° preoperatively to 105.1° postoperatively; the mean external rotation increased from 41° preoperatively to 101° postoperatively; the mean VAS for pain improved from 6.3 preoperatively to 2.2 postoperatively. In a longterm follow-up multicenter study, Favard et al.79) reported the 10-year survivorship of RTSA in 484 patients with rotator cuff arthropathy as 89%. However, they additionally mentioned that the Constant score decreased over time, and the 10-year survivorship fell to 72% when the endpoint was set as the Constant score of 30 points. By contrast, in a recent study, the 10-year survivorship was close to 90% in 46 cases of RTSA performed in relatively young patients (< 65 years) with a mean follow-up of 93 months. The patients also obtained good clinical outcomes, such as improvement in shoulder function scores and pain relief.80) However, given that RTSA is considered as the last resort, it should be carried out when all other salvage procedures have failed. Furthermore, if possible, it should be an option exclusively for elderly patients (≥ 65 years).
Irreparable RCTs unaccompanied by osteoarthritis can also be a primary indication for RTSA. Boileau et al.81) evaluated 42 shoulders that were managed by RTSA after failed rotator cuff surgery with a mean follow-up of 50 months. In pseudoparalytic shoulders, the mean active forward elevation increased from 56° preoperatively to 123° postoperatively, and complications occurred in 12%. Mulieri et al.82) also performed RTSA for the treatment of 72 irreparable RCTs without glenohumeral arthritis. During a mean 52 months of follow-up, shoulder function scores improved, pain was alleviated, and the complication rate was 20%. Zumstein et al.83) emphasized the need to resolve the ambiguity with regard to the definition of complications and reoperations among studies. They suggested to differentiate a problem as an event that does not affect the patient's final outcome from a complication that influences the outcome. We reviewed 21 different papers published between 1995 and 2008 to assess complications based on their definition. Among 782 shoulders, complications occurred in 188 shoulders (24%). The most common complication was instability (4.7%), followed by infection (3.8%) and glenoid loosening (3.5%). Although scapular notching (35%) was the most common event, it was classified as a problem according to the definition. However, considering that scapular notching could potentially be a complication, more long-term follow-up studies are needed to determine the validity of the definition of complication.
RTSA is a good option for a multitude of shoulder disorders. However, considering the various complications described in the literature, a cautious approach is advised when determining the procedure for the treatment of irreparable RCTs with pseudoparalysis unaccompanied by osteoarthritis. Oh et al.84) compared the outcomes of arthroscopic repair of large-to-massive RCTs between patients with and without pseudoparalysis using propensity score matching. In both patient groups, range of motion improved postoperatively. More significant improvement in active forward elevation was observed in the pseudoparalytic group. The function scores showed no significant difference between the groups, and pseudoparalysis was reversed in 73% of the pseudoparalytic patients after repair. Thus, they argued that pseudoparalysis should not be considered the absolute indication for RTSA. They suggested that considering the high complications rates of RTSA, rotator cuff repair should the first-line treatment option even in the case of nonarthritic large-to-massive RCTs with pseudoparalysis. Denard et al.8586) retrospectively reviewed arthroscopic rotator cuff repair of massive RCTs in pseudoparalytic patients who underwent either primary rotator cuff repair (group I, n = 39) or revision repair (group II, n = 14). Group I showed improvement in forward elevation (from a mean of 49° preoperatively to a mean of 155° postoperatively); the mean duration of pseudoparalysis was 4.6 months; and pseudoparalysis was reversed in 90% of patients. In group II, the mean forward elevation improved from 43° preoperatively to 109° postoperatively, the mean duration of pseudoparalysis was 20.8 months, and pseudoparalysis was reversed in 43%. In summary, the procedure led to reversal of preoperative pseudoparalysis in 90% of patients who had no previous surgery and the duration of pseudoparalysis was significantly shorter in them. In another prospective multicenter study, reversal of pseudoparalysis was observed in 95% of 56 massive RCT patients with pseudoparalysis. There was no difference in the rate of reversal of pseudoparalysis between patients with an AI of ≤ 7 mm and those with > 7-mm AI and between patients with fatty infiltration of grade 3 or higher and those with less than grade 3.86) Similarly, Miyazaki et al.87) reported a pseudoparalysis reversal rate of 97.4%. Based on these studies demonstrating a 90% likelihood of reversal of pseudoparalysis, we believe that rotator cuff repair is a more reasonable strategy than RTSA as the first line of treatment even in massive RCT patients with pseudoparalysis.
However, Werner et al.88) reported that clinical outcomes of RTSA were better when it was a primary treatment than a revision of a failed repair. In the study, of the total 58 irreparable RCT patients with pseudoparalysis, the procedure was the primary treatment in 17 and a revision in the remaining 41. At a mean follow-up of 38 months, the former group of patients had a lower revision rate and higher Constant scores. All these studies underscore the importance of a comprehensive decision-making process where a treatment decision is not based on the consideration of a few clinical presentations but on various factors, such as patient's age, level of activity, and comorbidities.
Factors affecting the outcome of RTSA surgery are still controversial. By medialization of the center of rotation, RTSA minimizes the torque of the glenoid components and recruits more fibers of deltoid muscle to act as shoulder abductors. However, there are some problems in medialization of the center of rotation, such as scapular notching, restriction of external rotation of the shoulder, and instability due to cam effect. Recently, lateralized glenoid components have been used to reduce scapular notching and improve passive internal rotation and external rotation. However, when using a lateralized glenoid component, there is a concern that the torque at the baseplate-glenoid interface increases and the shear force increases at the glenoid fixation site, thereby increasing the failure rate. The 135° neck shaft angle and the 155° neck shaft angle also have advantages and disadvantages. The 135° neck shaft angle is more anatomical, less scapular notching, and favorable for passive internal rotation and external rotation. The 155° neck shaft angle has good joint stability and is effective for lengthening deltoid muscle and improving ability of forward elevation. Repair of subscapularis with RTSA is controversial. Vourazeris et al.89) reported that primary RTSAs with or without subscapularis have similar clinical outcome scores, range of motion, strength, and rates of complications, including dislocations at 3 years of follow-up. Friedman et al.90) reported a significant increase in internal rotation in the subscapularis repair group compared to the non-subscapularis repair group. In addition, active abduction and passive external rotation were significantly increased in the non-subscapularis repair group. And no difference was noted in the complication or scapular notching rates between cohorts. Werner et al.91) reported that ASES scores were significantly less improved in patients who underwent subscapularis repair and glenosphere lateralization. The version of the humeral component plays an important role in range of motion and impingement in RTSA. Anteversion can significantly reduce the amount of external rotation that can be achieved after RTSA. Rhee et al.92) reported that range of motion after RTSA was not significantly different between 0° and 20° of humeral component retroversion angle and most daily activities did not differ between the two groups, but the activity score related to the internal rotation was better in the 0° retroversion angle group. The anatomical differences of the shoulders between Asian and Western populations should be considered: the Korean population has a smaller humerus length and a humerus head diameter than the Western population; the humerus neck shaft angle is similar between the two populations; the humerus retroversion is greater in the Korean population than in the Western population; and the Korean population has a smaller glenoid diameter and a larger lateral extension of acromion than the Western population.93) Because of these anatomical differences, it seems reasonable for Koreans to consider a lateralized prosthesis. Considering such differences between Asian and Western people as well as individual differences, it is necessary to implement an individualized approach.
Arthroplasty can be considered a first-line treatment for irreparable RCTs with advanced osteoarthritis. Hemiarthroplasty may provide satisfying outcomes in rotator cuff arthropathy patients without pseudoparalysis when the coracoacromial arch is intact. RTSA is the optimal solution among elderly patients with RCTs accompanied by pseudoparalysis and osteoarthritis for alleviation of pain and restoration of active elevation function. Factors that should be considered in arthroplasty include the patient's age and desired activity level, and presence of pseudoparalysis and osteoarthritis. For arthritic irreparable RCTs without pseudoparalysis, other procedures enabling joint preservation should be attempted first. In a treatment decision process, it is important to consider various relevant factors and have sufficient communication with patients to determine the most beneficial treatment strategy for them.
Proper treatment decisions for RCTs require a comprehensive analysis of multiple factors, including the patient's systemic medical condition, functional demands, extent and severity of tear, and previous history of shoulder surgery. For a patient with a poor systemic medical condition, conservative treatment aimed at pain relief can be an appropriate option. For elderly patients with low functional demands, conservative treatment or minimally invasive procedures, such as arthroscopic debridement or tuberoplasty, combined with partial repair can be effective. For relatively young patients with high functional demands, complete repair using various techniques can be attempted. If a complete repair is not feasible, patch graft augmentation, SCR, or balloon spacer insertion can be considered. Tendon transfer can also be recommended for young patients. An arthritic RCT may be successfully managed by RTSA.
Treatment of irreparable RCTs is a challenging task for orthopedic surgeons. It is imperative to determine an appropriate treatment strategy based on thorough assessment of various factors. We think that it is reasonable to consider arthroplasty for the treatment of irreparable RCTs accompanied by glenohumeral joint arthritis. However, repair should be the first-line treatment for nonarthritic RCTs. For elderly patients with pseudoparalysis or severe fatty infiltration, repair should also be the primary treatment option, if possible. All the treatment options described in this article should be considered for the maintenance of AI and restoration of force couples in repair of RCTs. In addition, further research needs to be conducted to explore methods that facilitate healing of rotator cuffs, such as biologic augmentation (platelet-rich plasma and adipose-derived stem cell) and systemic augmentation (cessation of smoking, vitamin D supplementation, treatment of osteoporosis, and management of diabetes and cholesterol), which will aid in improving treatment outcomes of irreparable RCTs.
Figures and Tables
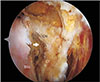 | Fig. 2Arthroscopic view of a right shoulder through a posterior portal using a 70° arthroscope. The coracohumeral ligament was released from the posterolateral arch of the coracoid process (arrow). CN: coracoid neck, CT: coracoid tip, SSc: subscapularis tendon. |
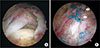 | Fig. 3(A) Arthroscopic image of a right shoulder with a massive rotator cuff tear. (B) Arthroscopic image obtained after rotator cuff repair and margin convergence (arrows). |
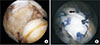 | Fig. 4(A) Arthroscopic image showing a massive rotator cuff tear. Repair was impossible because the rotator cuff was unable to be mobilized. (B) After allogenic dermal patch graft insertion, single-row repair was performed. The medial border of the graft (arrow) was sutured to the rotator cuff and a suture anchor was inserted at the lateral border of the graft. |
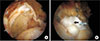 | Fig. 5(A) Arthroscopic image of a right shoulder with a massive rotator cuff tear. (B) Arthroscopic image obtained after rotator cuff repair and biceps augmentation (arrow). |
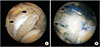 | Fig. 6(A) Arthroscopic image showing a massive rotator cuff tear. Superior capsular reconstruction involves acromioplasty for prevention of graft abrasion and preparation of superior aspect of the glenoid tubercle (arrows). (B) After allogenic graft insertion, the graft is attached medial to the superior aspect of the glenoid tubercle (arrows) and lateral to the greater tuberosity using one suture anchor. Then, the graft is sutured to the infraspinatus posteriorly and the subscapularis anteriorly to restore force coupling of the joint. |
 | Fig. 7Arthroscopic images of a right shoulder with a massive rotator cuff tear. The rotator cuff was unable to be mobilized; thus, following subacromial decompression (A), partial repair (black arrow) (B) and biodegradable subacromial spacer (white arrow; InSpace System, Orthospace, Caesarea, Israel) insertion (C) were performed. |
References
1. Chung SW, Kim JY, Kim MH, Kim SH, Oh JH. Arthroscopic repair of massive rotator cuff tears: outcome and analysis of factors associated with healing failure or poor postoperative function. Am J Sports Med. 2013; 41(7):1674–1683.
2. Warner JJ. Management of massive irreparable rotator cuff tears: the role of tendon transfer. Instr Course Lect. 2001; 50:63–71.
3. Neri BR, Chan KW, Kwon YW. Management of massive and irreparable rotator cuff tears. J Shoulder Elbow Surg. 2009; 18(5):808–818.

4. Levy O, Mullett H, Roberts S, Copeland S. The role of anterior deltoid reeducation in patients with massive irreparable degenerative rotator cuff tears. J Shoulder Elbow Surg. 2008; 17(6):863–870.

5. Yian EH, Sodl JF, Dionysian E, Schneeberger AG. Anterior deltoid reeducation for irreparable rotator cuff tears revisited. J Shoulder Elbow Surg. 2017; 26(9):1562–1565.

6. Gialanella B, Bertolinelli M. Corticosteroids injection in rotator cuff tears in elderly patient: pain outcome prediction. Geriatr Gerontol Int. 2013; 13(4):993–1001.

7. Maman E, Yehuda C, Pritsch T, et al. Detrimental effect of repeated and single subacromial corticosteroid injections on the intact and injured rotator cuff: a biomechanical and imaging study in rats. Am J Sports Med. 2016; 44(1):177–182.

8. Rockwood CA Jr, Williams GR Jr, Burkhead WZ Jr. Debridement of degenerative, irreparable lesions of the rotator cuff. J Bone Joint Surg Am. 1995; 77(6):857–866.
9. Kempf JF, Gleyze P, Bonnomet F, et al. A multicenter study of 210 rotator cuff tears treated by arthroscopic acromioplasty. Arthroscopy. 1999; 15(1):56–66.

10. Veado MA, Rodrigues AU. Functional evaluation of patients who have undergone arthroscopic debridement to treat massive and irreparable tears of the rotator cuff. Rev Bras Ortop. 2010; 45(5):426–431.

11. Fenlin JM Jr, Chase JM, Rushton SA, Frieman BG. Tuberoplasty: creation of an acromiohumeral articulation-a treatment option for massive, irreparable rotator cuff tears. J Shoulder Elbow Surg. 2002; 11(2):136–142.

12. Scheibel M, Lichtenberg S, Habermeyer P. Reversed arthroscopic subacromial decompression for massive rotator cuff tears. J Shoulder Elbow Surg. 2004; 13(3):272–278.

13. Verhelst L, Vandekerckhove PJ, Sergeant G, Liekens K, Van Hoonacker P, Berghs B. Reversed arthroscopic subacromial decompression for symptomatic irreparable rotator cuff tears: mid-term follow-up results in 34 shoulders. J Shoulder Elbow Surg. 2010; 19(4):601–608.

14. Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011; 27(10):1341–1350.

15. Park JG, Cho NS, Song JH, Baek JH, Rhee YG. Long-term outcome of tuberoplasty for irreparable massive rotator cuff tears: is tuberoplasty really applicable? J Shoulder Elbow Surg. 2016; 25(2):224–231.

16. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder's “suspension bridge”. Arthroscopy. 1993; 9(6):611–616.

17. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994; 10(4):363–370.

18. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012; 28(6):761–768.

19. Galasso O, Riccelli DA, De Gori M, et al. Quality of life and functional results of arthroscopic partial repair of irreparable rotator cuff tears. Arthroscopy. 2017; 33(2):261–268.

20. Iagulli ND, Field LD, Hobgood ER, Ramsey JR, Savoie FH 3rd. Comparison of partial versus complete arthroscopic repair of massive rotator cuff tears. Am J Sports Med. 2012; 40(5):1022–1026.

21. Berth A, Neumann W, Awiszus F, Pap G. Massive rotator cuff tears: functional outcome after debridement or arthroscopic partial repair. J Orthop Traumatol. 2010; 11(1):13–20.

22. Oh JH, McGarry MH, Jun BJ, et al. Restoration of shoulder biomechanics according to degree of repair completion in a cadaveric model of massive rotator cuff tear: importance of margin convergence and posterior cuff fixation. Am J Sports Med. 2012; 40(11):2448–2453.

23. Bigliani LU, Cordasco FA, McLlveen SJ, Musso ES. Operative repair of massive rotator cuff tears: long-term results. J Shoulder Elbow Surg. 1992; 1(3):120–130.

24. Tauro JC. Arthroscopic “interval slide” in the repair of large rotator cuff tears. Arthroscopy. 1999; 15(5):527–530.

25. Anley CM, Chan SK, Snow M. Arthroscopic treatment options for irreparable rotator cuff tears of the shoulder. World J Orthop. 2014; 5(5):557–565.

26. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004; 20(1):22–33.

27. Kim SJ, Kim SH, Lee SK, Seo JW, Chun YM. Arthroscopic repair of massive contracted rotator cuff tears: aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Joint Surg Am. 2013; 95(16):1482–1488.
28. Berdusco R, Trantalis JN, Nelson AA, et al. Arthroscopic repair of massive, contracted, immobile tears using interval slides: clinical and MRI structural follow-up. Knee Surg Sports Traumatol Arthrosc. 2015; 23(2):502–507.

29. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996; 12(3):335–338.

30. Burkhart SS. The principle of margin convergence in rotator cuff repair as a means of strain reduction at the tear margin. Ann Biomed Eng. 2004; 32(1):166–170.

31. Shindle MK, Nho SJ, Nam D, et al. Technique for margin convergence in rotator cuff repair. HSS J. 2011; 7(3):208–212.

32. Mazzocca AD, Bollier M, Fehsenfeld D, et al. Biomechanical evaluation of margin convergence. Arthroscopy. 2011; 27(3):330–338.

33. Liu J, Hughes RE, O'Driscoll SW, An KN. Biomechanical effect of medial advancement of the supraspinatus tendon: a study in cadavera. J Bone Joint Surg Am. 1998; 80(6):853–859.

34. Yamamoto N, Itoi E, Tuoheti Y, et al. Glenohumeral joint motion after medial shift of the attachment site of the supraspinatus tendon: a cadaveric study. J Shoulder Elbow Surg. 2007; 16(3):373–378.

35. Kim YK, Jung KH, Won JS, Cho SH. Medialized repair for retracted rotator cuff tears. J Shoulder Elbow Surg. 2017; 26(8):1432–1440.

36. Soler JA, Gidwani S, Curtis MJ. Early complications from the use of porcine dermal collagen implants (Permacol) as bridging constructs in the repair of massive rotator cuff tears: a report of 4 cases. Acta Orthop Belg. 2007; 73(4):432–436.
37. Sclamberg SG, Tibone JE, Itamura JM, Kasraeian S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elbow Surg. 2004; 13(5):538–541.

38. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears: a randomized, controlled trial. J Bone Joint Surg Am. 2006; 88(6):1238–1244.

39. Encalada-Diaz I, Cole BJ, Macgillivray JD, et al. Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: preliminary results at 12 months' follow-up. J Shoulder Elbow Surg. 2011; 20(5):788–794.

40. Ciampi P, Scotti C, Nonis A, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014; 42(5):1169–1175.

41. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978; 60(5):681–684.

42. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Relat Res. 1988; (228):218–226.

43. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006; 34(3):392–396.

44. Barber FA, Burns JP, Deutsch A, Labbe MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012; 28(1):8–15.

45. Rhee SM, Oh JH. Bridging graft in irreparable massive rotator cuff tears: Autogenic Biceps Graft versus Allogenic Dermal Patch Graft. Clin Orthop Surg. 2017; 9(4):497–505.

46. Yoon JP, Chung SW, Kim JY, et al. Outcomes of combined bone marrow stimulation and patch augmentation for massive rotator cuff tears. Am J Sports Med. 2016; 44(4):963–971.

47. Chung SW, Song BW, Kim YH, Park KU, Oh JH. Effect of platelet-rich plasma and porcine dermal collagen graft augmentation for rotator cuff healing in a rabbit model. Am J Sports Med. 2013; 41(12):2909–2918.

48. Neviaser JS. Ruptures of the rotator cuff of the shoulder: new concepts in the diagnosis and operative treatment of chronic ruptures. Arch Surg. 1971; 102(5):483–485.
49. Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008; 36(8):1511–1518.

50. Cho NS, Yi JW, Rhee YG. Arthroscopic biceps augmentation for avoiding undue tension in repair of massive rotator cuff tears. Arthroscopy. 2009; 25(2):183–191.

51. Ji JH, Shafi M, Jeong JJ, Park SE. Arthroscopic repair of large and massive rotator cuff tears using the biceps-incorporating technique: mid-term clinical and anatomical results. Eur J Orthop Surg Traumatol. 2014; 24(8):1367–1374.

52. Oh JH, Tilan J, Chen YJ, Chung KC, McGarry MH, Lee TQ. Biomechanical effect of latissimus dorsi tendon transfer for irreparable massive cuff tear. J Shoulder Elbow Surg. 2013; 22(2):150–157.

53. Aoki M, Okamura K, Fukushima S, Takahashi T, Ogino T. Transfer of latissimus dorsi for irreparable rotator-cuff tears. J Bone Joint Surg Br. 1996; 78(5):761–766.

54. Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg Am. 2006; 88(1):113–120.

55. Werner CM, Zingg PO, Lie D, Jacob HA, Gerber C. The biomechanical role of the subscapularis in latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Shoulder Elbow Surg. 2006; 15(6):736–742.

56. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015; 31(4):599–607.e1.

57. El-Azab HM, Rott O, Irlenbusch U. Long-term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2015; 97(6):462–469.

58. Henseler JF, Nagels J, Nelissen RG, de Groot JH. Does the latissimus dorsi tendon transfer for massive rotator cuff tears remain active postoperatively and restore active external rotation? J Shoulder Elbow Surg. 2014; 23(4):553–560.

59. Resch H, Povacz P, Ritter E, Matschi W. Transfer of the pectoralis major muscle for the treatment of irreparable rupture of the subscapularis tendon. J Bone Joint Surg Am. 2000; 82(3):372–382.

60. Gavriilidis I, Kircher J, Magosch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010; 34(5):689–694.

61. Jost B, Puskas GJ, Lustenberger A, Gerber C. Outcome of pectoralis major transfer for the treatment of irreparable subscapularis tears. J Bone Joint Surg Am. 2003; 85(10):1944–1951.

62. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012; 40(10):2248–2255.

63. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013; 29(3):459–470.

64. Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. arthroscopy. 2018; 34(1):93–99.

65. Senekovic V, Poberaj B, Kovacic L, et al. The biodegradable spacer as a novel treatment modality for massive rotator cuff tears: a prospective study with 5-year follow-up. Arch Orthop Trauma Surg. 2017; 137(1):95–103.

66. Holschen M, Brand F, Agneskirchner JD. Subacromial spacer implantation for massive rotator cuff tears: clinical outcome of arthroscopically treated patients. Obere Extrem. 2017; 12(1):38–45.
67. Deranlot J, Herisson O, Nourissat G, et al. Arthroscopic subacromial spacer implantation in patients with massive irreparable rotator cuff tears: clinical and radiographic results of 39 retrospectives cases. Arthroscopy. 2017; 33(9):1639–1644.

68. Gervasi E, Cautero E, Dekel A. Fluoroscopy-guided implantation of subacromial “biodegradable spacer” using local anesthesia in patients with irreparable rotator cuff tear. Arthrosc Tech. 2014; 3(4):e455–e458.

69. Savarese E, Romeo R. New solution for massive, irreparable rotator cuff tears: the subacromial “biodegradable spacer”. Arthrosc Tech. 2012; 1(1):e69–e74.

70. Senekovic V, Poberaj B, Kovacic L, Mikek M, Adar E, Dekel A. Prospective clinical study of a novel biodegradable subacromial spacer in treatment of massive irreparable rotator cuff tears. Eur J Orthop Surg Traumatol. 2013; 23(3):311–316.

71. Bozkurt M, Akkaya M, Gursoy S, Isik C. Augmented fixation with biodegradable subacromial spacer after repair of massive rotator cuff tear. Arthrosc Tech. 2015; 4(5):e471–e474.

72. Goldberg SS, Bell JE, Kim HJ, Bak SF, Levine WN, Bigliani LU. Hemiarthroplasty for the rotator cuff-deficient shoulder. J Bone Joint Surg Am. 2008; 90(3):554–559.

73. Zuckerman JD, Scott AJ, Gallagher MA. Hemiarthroplasty for cuff tear arthropathy. J Shoulder Elbow Surg. 2000; 9(3):169–172.

74. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004; 86:Suppl 2. 35–40.
75. Sanchez-Sotelo J, Cofield RH, Rowland CM. Shoulder hemiarthroplasty for glenohumeral arthritis associated with severe rotator cuff deficiency. J Bone Joint Surg Am. 2001; 83(12):1814–1822.

76. Baulot E, Sirveaux F, Boileau P. Grammont's idea: the story of Paul Grammont's functional surgery concept and the development of the reverse principle. Clin Orthop Relat Res. 2011; 469(9):2425–2431.

77. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004; 86(3):388–395.
78. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency: a minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006; 88:Suppl 1 Pt 2. 178–190.

79. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011; 469(9):2469–2475.

80. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013; 22(9):1199–1208.

81. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009; 18(4):600–606.

82. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010; 92(15):2544–2556.

83. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011; 20(1):146–157.

84. Oh JH, Kim SH, Shin SH, et al. Outcome of rotator cuff repair in large-to-massive tear with pseudoparalysis: a comparative study with propensity score matching. Am J Sports Med. 2011; 39(7):1413–1420.

85. Denard PJ, Ladermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012; 28(9):1214–1219.

86. Denard PJ, Ladermann A, Brady PC, et al. Pseudoparalysis from a massive rotator cuff tear is reliably reversed with an arthroscopic rotator cuff repair in patients without preoperative glenohumeral arthritis. Am J Sports Med. 2015; 43(10):2373–2378.

87. Miyazaki AN, Fregoneze M, Santos PD, et al. Functional evaluation of arthroscopic repair of rotator cuff injuries in patients with pseudoparalysis. Rev Bras Ortop. 2014; 49(2):178–182.

88. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005; 87(7):1476–1486.

89. Vourazeris JD, Wright TW, Struk AM, King JJ, Farmer KW. Primary reverse total shoulder arthroplasty outcomes in patients with subscapularis repair versus tenotomy. J Shoulder Elbow Surg. 2017; 26(3):450–457.

90. Friedman RJ, Flurin PH, Wright TW, Zuckerman JD, Roche CP. Comparison of reverse total shoulder arthroplasty outcomes with and without subscapularis repair. J Shoulder Elbow Surg. 2017; 26(4):662–668.

91. Werner BC, Wong AC, Mahony GT, et al. Clinical outcomes after reverse shoulder arthroplasty with and without subscapularis repair: the importance of considering glenosphere lateralization. J Am Acad Orthop Surg. 2018; 26(5):e114–e119.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download