1. Bang HS, Seo DY, Chung YM, Oh KM, Park JJ, Arturo F, Jeong SH, Kim N, Han J. Ursolic Acid-induced elevation of serum irisin augments muscle strength during resistance training in men. Korean J Physiol Pharmacol. 2014; 18:441–446. PMID:
25352765.

2. Kim JS, Kim MG, Kang SW, Kim BR, Beak MY, Park YM, Shin MS. Association of obesity, hypertension and their impact on cardiac function and exercise capacity. Korean Circ J. 2016; 46:e51.
3. Jung SR, Kim KJ. Exercise-induced PGC-1 transcriptional factors in skeletal muscle. Integr Med Res. 2014; 3:155–160. PMID:
28664092.

4. Constable SH, Favier RJ, McLane JA, Fell RD, Chen M, Holloszy JO. Energy metabolism in contracting rat skeletal muscle: adaptation to exercise training. Am J Physiol. 1987; 253:C316–C322. PMID:
3618765.

5. Takada S, Kinugawa S, Matsushima S, Takemoto D, Furihata T, Mizushima W, Fukushima A, Yokota T, Ono Y, Shibata H, Okita K, Tsutsui H. Sesamin prevents decline in exercise capacity and impairment of skeletal muscle mitochondrial function in mice with high-fat diet-induced diabetes. Exp Physiol. 2015; 11:1319–1330.

6. Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011; 58:1780–1791. PMID:
21996391.
7. Lim CL, Mackinnon LT. The roles of exercise-induced immune system disturbances in the pathology of heat stroke: the dual pathway model of heat stroke. Sports Med. 2006; 36:39–64. PMID:
16445310.
8. Cook BJ, Hausenblas HA. The role of exercise dependence for the relationship between exercise behavior and eating pathology: mediator or moderator? J Health Psychol. 2008; 13:495–502. PMID:
18420757.
9. Sprangers RL, Wesseling KH, Imholz AL, Imholz BP, Wieling W. Initial blood pressure fall on stand up and exercise explained by changes in total peripheral resistance. J Appl Physiol (1985). 1991; 70:523–530. PMID:
2022542.

10. Ubeda Tikkanen A, Opotowsky AR, Bhatt AB, Landzberg MJ, Rhodes J. Physical activity is associated with improved aerobic exercise capacity over time in adults with congenital heart disease. Int J Cardiol. 2013; 168:4685–4691. PMID:
23962775.

11. Seo DY, Lee SR, Kim N, Ko KS, Rhee BD, Han J. Humanized animal exercise model for clinical implication. Pflugers Arch. 2014; 466:1673–1687. PMID:
24647666.

12. Itoh Y, Hayakawa K, Mori T, Agata N, Inoue-Miyazu M, Murakami T, Sokabe M, Kawakami K. Stand-up exercise training facilitates muscle recovery from disuse atrophy by stimulating myogenic satellite cell proliferation in mice. Physiol Rep. 2014; DOI:
10.14814/phy2.12185.

13. Helmreich DL, Parfitt DB, Lu XY, Akil H, Watson SJ. Relation between the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during repeated stress. Neuroendocrinology. 2005; 81:183–192. PMID:
16020927.

14. Seo DY, McGregor RA, Noh SJ, Choi SJ, Mishchenko NP, Fedoreyev SA, Stonik VA, Han J. Echinochrome A improves exercise capacity during short-term endurance training in rats. Mar Drugs. 2015; 13:5722–5731. PMID:
26371013.
15. Jeffery NS, Stephenson RS, Gallagher JA, Jarvis JC, Cox PG. Micro-computed tomography with iodine staining resolves the arrangement of muscle fibres. J Biomech. 2011; 44:189–192. PMID:
20846653.

16. Bruns RF, Menegatti CM, Martins WP, Araujo Junior E. Applicability of pocket ultrasound during the first trimester of pregnancy. Med Ultrason. 2015; 17:284–288. PMID:
26343074.

17. Fukada S, Morikawa D, Yamamoto Y, Yoshida T, Sumie N, Yamaguchi M, Ito T, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. Genetic background affects properties of satellite cells and mdx phenotypes. Am J Pathol. 2010; 176:2414–2424. PMID:
20304955.

18. Brenner DA, Apstein CS, Saupe KW. Exercise training attenuates age-associated diastolic dysfunction in rats. Circulation. 2001; 104:221–226. PMID:
11447090.

19. Reznik M. Electron microscopy and histochemistry of regenerating skeletal muscle. Neurol India. 1972; 20:71–74. PMID:
4264095.
20. Ahmet I, Tae HJ, de Cabo R, Lakatta EG, Talan MI. Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol. 2011; 51:263–271. PMID:
21586294.

21. Zhang B, Wang B, Cao S, Wang Y. Epigallocatechin-3-Gallate (EGCG) attenuates traumatic brain injury by inhibition of edema formation and oxidative stress. Korean J Physiol Pharmacol. 2015; 19:491–497. PMID:
26557015.

22. Andersson C, Lyass A, Larson MG, Spartano NL, Vita JA, Benjamin EJ, Murabito JM, Esliger DW, Blease SJ, Hamburg NM, Mitchell GF, Vasan RS. Physical activity measured by accelerometry and its associations with cardiac structure and vascular function in young and middle-aged adults. J Am Heart Assoc. 2015; 4:e001528. PMID:
25792127.

23. Harris L, Hankey C, Murray H, Melville C. The effects of physical activity interventions on preventing weight gain and the effects on body composition in young adults with intellectual disabilities: systematic review and meta-analysis of randomized controlled trials. Clin Obes. 2015; 5:198–210. PMID:
26126951.

24. Chen N, Li Q, Liu J, Jia S. Irisin, an exercise-induced myokine as a metabolic regulator: an updated narrative review. Diabetes Metab Res Rev. 2016; 32:51–59. PMID:
25952527.

25. Mifune H, Tajiri Y, Nishi Y, Hara K, Iwata S, Tokubuchi I, Mitsuzono R, Yamada K, Kojima M. Voluntary exercise contributed to an amelioration of abnormal feeding behavior, locomotor activity and ghrelin production concomitantly with a weight reduction in high fat diet-induced obese rats. Peptides. 2015; 71:49–55. PMID:
26122892.

26. Mahajan RD, Patra SK. Irisin, a novel myokine responsible for exercise induced browning of white adipose tissue. Indian J Clin Biochem. 2013; 28:102–103. PMID:
24381432.

27. Bluher S, Panagiotou G, Petroff D, Markert J, Wagner A, Klemm T, Filippaios A, Keller A, Mantzoros CS. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity (Silver Spring). 2014; 22:1701–1708. PMID:
24644099.
28. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012; 481:463–468. PMID:
22237023.
29. Al-Daghri NM, Alokail MS, Rahman S, Amer OE, Al-Attas OS, Alfawaz H, Tripathi G, Sabico S, Chrousos GP, McTernan PG, Piya MK. Habitual physical activity is associated with circulating irisin in healthy controls but not in subjects with diabetes mellitus type 2. Eur J Clin Invest. 2015; 45:775–781. PMID:
26011590.

30. Hew-Butler T, Landis-Piwowar K, Byrd G, Seimer M, Seigneurie N, Byrd B, Muzik O. Plasma irisin in runners and nonrunners: no favorable metabolic associations in humans. Physiol Rep. 2015; DOI:
10.14814/phy2.12262.

31. Hecksteden A, Wegmann M, Steffen A, Kraushaar J, Morsch A, Ruppenthal S, Kaestner L, Meyer T. Irisin and exercise training in humans results from a randomized controlled training trial. BMC Med. 2013; 11:235. PMID:
24191966.

32. Gomez-Merino D, Drogou C, Guezennec CY, Chennaoui M. Effects of chronic exercise on cytokine production in white adipose tissue and skeletal muscle of rats. Cytokine. 2007; 40:23–29. PMID:
17826174.

33. Yang X, Zhou Y, Wang P, He C, He H. Effects of whole body vibration on pulmonary function, functional exercise capacity and quality of life in people with chronic obstructive pulmonary disease: A systematic review. Clin Rehabil. 2015; DOI:
10.1177/02692155155-89202. [Epub ahead of print].

34. Nishikawa M, Ishimori N, Takada S, Saito A, Kadoguchi T, Furihata T, Fukushima A, Matsushima S, Yokota T, Kinugawa S, Tsutsui H. AST-120 ameliorates lowered exercise capacity and mitochondrial biogenesis in the skeletal muscle from mice with chronic kidney disease via reducing oxidative stress. Nephrol Dial Transplant. 2015; 30:934–942. PMID:
25878055.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


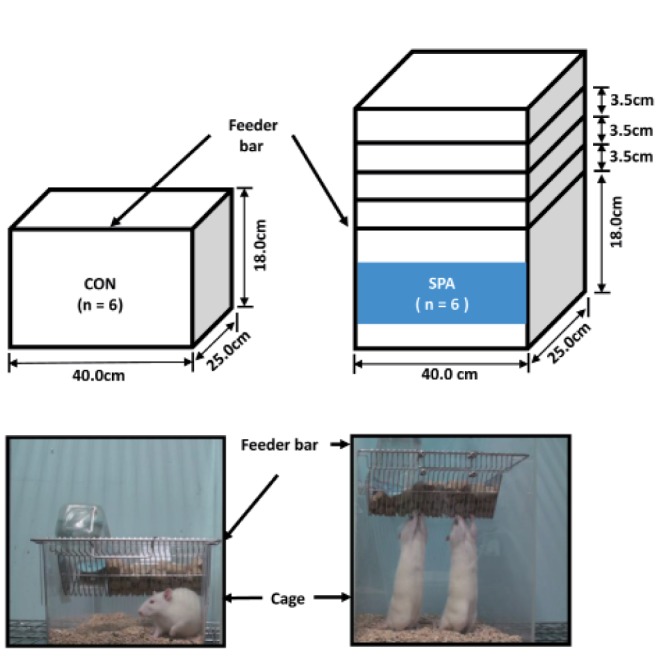
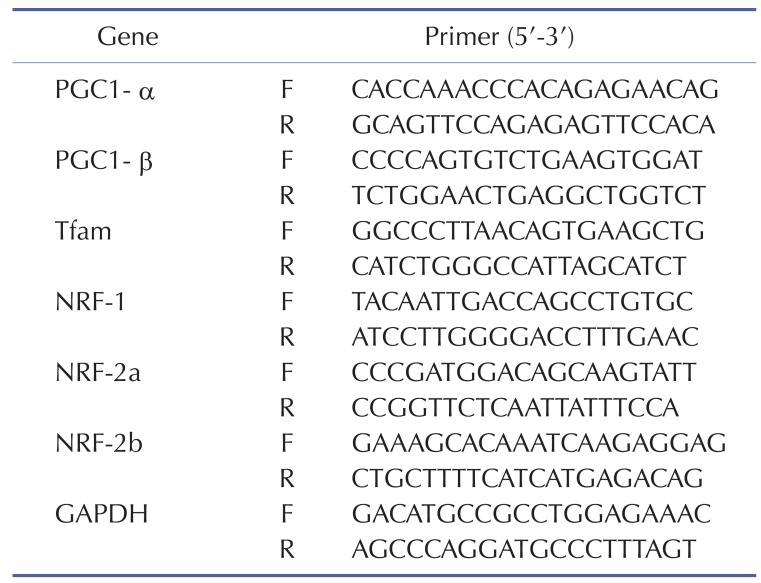
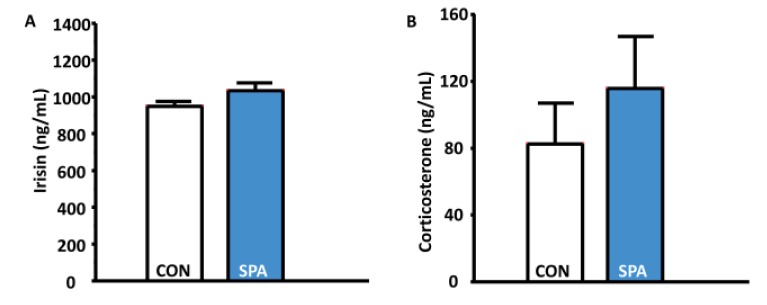
 XML Download
XML Download