Abstract
Objective
The purpose of this study is to estimate the risk of postoperative lymphocele development after lymphadenectomy in gynecologic cancer patients through establishing a nomogram.
Methods
We retrospectively reviewed 371 consecutive gynecologic cancer patients undergoing lymphadenectomy between 2009 and 2014. Association of the development of postoperative lymphocele with clinical characteristics was evaluated in univariate and multivariate regression analyses. Nomograms were built based on the data of multivariate analysis using R-software.
Results
Mean age at the operation was 50.8±11.1 years. Postoperative lymphocele was found in 70 (18.9%) patients. Of them, 22 (31.4%) had complicated one. Multivariate analysis revealed that hypertension (hazard ratio [HR], 3.0; 95% confidence interval [CI], 1.5 to 6.0; P=0.003), open surgery (HR, 3.2; 95% CI, 1.4 to 7.1; P=0.004), retrieved lymph nodes (LNs) >21 (HR, 1.8; 95% CI, 1.0 to 3.3; P=0.042), and no use of intermittent pneumatic compression (HR, 2.7; 95% CI, 1.0 to 7.2; P=0.047) were independent risk factors for the development of postoperative lymphocele. The nomogram appeared to be accurate and predicted the lymphocele development better than chance (concordance index, 0.754). For complicated lymphoceles, most variables which have shown significant association with general lymphocele lost the statistical significance, except hypertension (P=0.011) and mean number of retrieved LNs (29.5 vs. 21.1; P=0.001). A nomogram for complicated lymphocele showed similar predictive accuracy (concordance index, 0.727).
Pelvic and/or para-aortic lymphadenectomy is an essential part of surgical treatment for most of the patients with gynecologic malignancy. Comprehensive staging including lymph node (LN) dissection enables physicians to establish the best possible treatment plan for minimizing tumor recurrence. However, patients who underwent pelvic and/or para-aortic lymphadenectomy often suffer from various complications including bleeding, postoperative ileus, lymphedema, and lymphocele [1].
A lymphocele, which is defined as a collection of lymphatic fluid in the retroperitoneal space, is one of the most common complications of pelvic lymphadenectomy in patients with gynecologic malignancies [2]. Most lymphoceles are small asymptomatic and of little clinical significance [1]. However, patients with larger ones may sometimes have embarrassing symptoms, such as, lower abdominal pain, constipation, urinary frequency and edema of the genitalia or lower extremities. Worse than those, serious sequelae, including infection of lymphocele, hydronephrosis, intestinal obstruction, deep vein thrombosis, and pulmonary embolism could also be followed [13]. In those conditions, more aggressive interventions, including needle aspiration, percutaneous catheter drainage with or without sclerotherapy, and surgical marsupialization may be required [4].
Theoretically, lymphoceles could be developed in anyone after surgical closure of afferent lymphatic vessels from the site of lymphadenectomy. Not all, however, only some patients, reportedly 1% to 49% of patients who underwent pelvic lymphadenectomy for the treatment of gynecologic malignancy [25], developed postoperative lymphoceles. There are several risk factors reported that could contribute to post-lymphadenectomy lymphocele formation. Mori [6] noted that the extent of lymphadenectomy was correlated with the development of a lymphocyst. High body mass index (BMI), the presence of metastatic disease in LNs, postoperative adjuvant radiation therapy (RT), preoperative heparinization, use of retroperitoneal tube drainage and peritonization were also reported as risk factors [2578910]. However, tumor stage or histology was not associated with lymphocele formation [1].
Despite those reported risk factors of postoperative lymphoceles, possibility of the development of lymphoceles, especially for large complicated ones, is difficult to be quantified because each individual risk factor has never been integrated into one risk-estimation system. The purpose of this study, therefore, is to estimate the risk of postoperative lymphocele development after pelvic and/or para-aortic LN dissection in gynecologic cancer patients through establishing a nomogram.
We retrospectively reviewed medical records of 371 consecutive gynecologic cancer patients undergoing pelvic and/or para-aortic lymphadenectomy for the treatment of gynecologic cancer in Seoul National University Bundang Hospital between 2009 and 2014. Of them, 146 (39.4%) had cervical cancer, 111 (29.9%) uterine corpus cancer, 109 (29.4%) ovarian cancer, and 5 (1.3%) had other gynecologic malignancies. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-1504-296-117).
Lymphocele was defined as a newly developed cystic lesion at lymphadenectomy sites in postoperative follow-up images including computed tomography (CT), magnetic resonance imaging (MRI), or ultrasonography within 2 years after the operation, but not likely a tumor recurrence when clinical situation was correlated. Size of the lymphocle was calculated as a single deepest diameter in the largest lymphocele. Any lymphocele >1 cm was included in this study. The lymphocele was counted as a complicated one when a procedural management including aspiration or percutaneous catheter drainage with or without sclerotherapy was performed.
In addition to basic demographic data including age and BMI at the operation, menopause, hypertension, and diabetes, data of clinicopathologic variables that could be associated with the development of postoperative lymphocele were collected: origin of cancer (cervical cancer, uterine cancer, ovarian cancer, and others), stage, surgery type (open vs. laparoscopy), number of LNs dissected, LN metastasis, operation time, duration of surgical drain indwelling, use of intermittent pneumatic compression (IPC) or anti-embolic stocking, and start of ambulation after surgery, and postoperative adjuvant RT. The criterion of drain removal was total drain amount <50 mL per day for 2 days.
All variables were compared between patients with and without lymphocele using student's t-test and χ2 test for continuous and categorical variables, respectively. Univariate and multivariate regression analyses were performed to identify risk factors for postoperative lymphocele. Variables with P<0.25 in univariate analysis were selected to enter multivariate analysis. SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used. P<0.05 was considered statistically significant. Nomograms were built on the basis of the information obtained on multivariate analysis. Its statistical performance was evaluated by discrimination and calibration, bootstrap corrected concordance index computed as the measure of discrimination, and calibration assessed by comparing predicted vs. observed probability of postoperative lymphocele development. Then, 1,000 bootstrap resamples were used to reduce overfit bias. All statistical analyses were performed using R 3.1.1. (rms package; R Project, Vienna, Austria).
Mean age at the operation was 50.8±11.1 years. Postoperative lymphocele was found in 70 (18.9%) patients. Of them, 22 (31.4%) had complicated lymphoceles that needed intervention for symptom improvement or size reduction. Diagnosis of lymphocele was not different according to the primary cancer type: 16.4%, 17.1%, 23.9%, and 20.0% in patients with uterine cervix, corpus, ovary, and other gynecologic malignancies, respectively.
Table 1 shows patient characteristics and their associations with postoperative lymphocele. Hypertension (P=0.025), open surgery compared with laparoscopic surgery (P<0.001), LN metastasis (P=0.010), and no use of perioperative IPC (P=0.008) or anti-embolic stocking (P=0.002) were significantly associated with postoperative lymphocele development. Number of retrieved LN was significantly higher in patients with lymphocele than those without lymphocele (mean, 26.4 vs. 20.5; P<0.001). Long placement of surgical drain >4 days was also associated with postoperative lymphocele (P=0.005), however, mean duration of surgical drain indwelling was not different between lymphocele-positive and -negative women (6.6±3.7 vs. 5.9±4.6 days; P=0.275; data not shown). Age and BMI were not different between the 2 groups, either. Advanced stage, extent of lymphadenectomy (combined para-aortic and pelvic lymphadenectomy vs. pelvic lymphadenectomy alone), late postoperative ambulation >24hr after surgery, and postoperative adjuvant RT were not associated with lymphocele.
Only for complicated lymphoceles (Table 2), most variables which have shown significant association with lymphocele in Table 1 lost the statistical significance, except hypertension (P=0.011) and mean number of retrieved LNs (29.5 vs. 21.1; P=0.001). Compared with non-complicated lymphocele, complicated lymphocele tended to occur more frequently in ovarian cancer (10.0%) and other gynecologic malignancies including vulvar cancer (20.0%) than in cervical (2.7%) or uterine corpus cancer (5.4%) (P=0.067; data not shown). Of note, size of the majority of complicated lymphocele (86.4%) was greater than 5 cm whereas only 2.9% of non-complicated lymphocele was 5 cm or more (P<0.001; data not shown).
Multivariate analysis revealed that hypertension (hazard ratio [HR], 3.0; 95% confidence interval [CI], 1.5 to 6.0; P=0.003), open surgery (HR, 3.2; 95% CI, 1.4 to 7.1; P=0.004), retrieved LN >21 (HR, 1.8; 95% CI, 1.0 to 3.3; P=0.042), and no use of IPC (HR, 2.7; 95% CI, 1.0 to 7.2; P=0.047) were independent risk factors for the development of postoperative lymphocele (Table 3). Those variables entering multivariate regression analysis were used for building up a nomogram to calculate the probability of the development of postoperative lymphocele (Fig. 1). The concordance index of the nomogram was 0.754 (Fig. 2). The calibration plot of the nomogram showed good agreement between predictions and observations (Fig. 3). Predictions for the groups between 12% and 30% tended to be underestimated, while those over 30% were slightly overestimated.
On the other hand, complicated lymphocele was independently associated with only hypertension (HR, 3.7; 95% CI, 1.4 to 9.7; P=0.007) and high number of retrieved LN >21 (HR, 2.9; 95% CI, 1.1 to 8.0; P=0.034) (Supplementary Table 1). The concordance index of the nomogram was 0.727 (data not shown).
Our study demonstrated that hypertension, open surgery compared with laparoscopic approach, high number of retrieved LN, and no use of perioperative IPC were independent risk factors for the development of postoperative lymphocele in patients who underwent lymphadenectomy for the treatment of gynecologic cancer. Of those, in particular, hypertension and high number of retrieved LN were also risk factors for complicated lymphocele which needs intervention. Nomograms may predict the possibility of lymphocele development. For example, a woman with ovarian cancer, who underwent open staging operation including lymphadenectomy (100 points), had hypertension (90 points), had 30 LNs retrieved during lymphadenectomy but no LN metastasis (50 points), had surgical drain only for 3 days after surgery (0 point), used both of anti-embolic stocking and IPC (0 point), and ambulation started from 2nd postoperative day, has total score of 240 and the probability of development of postoperative lymphocele is 20% (Fig. 1). In this woman, the probability of complicated lymphocele is estimated around 10% (Supplementary Fig. 1).
There were lots of reports of risk factors for the occurrence of lymphocele even though they were inconsistent. High BMI was significantly associated with lymphocele in the study of Kim et al. [4], however, it was not the case in other studies including ours [2]. The association of type of tumor and adjuvant RT with lymphocele was also inconsistently reported between studies [241112]. Even high number of retrieved LN, which was the most commonly reported risk factor, was not shown to be associated with lymphocele formation in the study of Tam et al. [12]. Placement of suction drains with the peritoneum open was reported to increase the risk of lymphocyst formation with related symptoms in a systematic review [5]. To the best of our knowledge, however, the possibility of lymphocele development has not been evaluated before as a function of how long she had the surgical drain. Although mean duration of drain placement was not different between lymphocele-positive and -negative patients in our study (Table 1), median duration was significantly longer in patients with lymphocele than those without lymphocele (6.0 vs. 4.0 days; P=0.030). Thus, long indwelling of surgical drain was incorporated into variables for building a nomogram even though it failed to show a statistical significance in multivariate analysis. Hypertension was demonstrated for the first time in our study as an independent risk factor for the development of lymphocele including complicated ones. The plausible explanations include increased leakage of lymphatic fluid through the disrupted lymphatic channels by secondary overpressure due to the systemic hypertension.
To the best of our knowledge, this is the first study demonstrating clinical utility of a nomogram for predicting the possibility of lymphocele development in post-lymphadenectomy patients with gynecologic cancer. We tried to build up another nomogram for predicting complicated lymphoceles. It is noteworthy that most lymphoceles are reported to occur within 3–8 weeks [4], during which postoperative adjuvant treatment, if indicated, is supposed to start. Complicated lymphoceles can affect quality of life but also potentially compromise the success of cancer treatment by delaying planned treatment such as chemotherapy and/or RT [3]. The incidence of complicated lymphocele of our study was 5.9% (22 out of 371), which was compatible with previous reported range of infected lymphocele (2.1% to 15.3%) [11213]. However, there were only a few independent risk factors for complicated lymphocele in a multivariate regression analysis and, therefore, the corresponding nomogram for predicting complicated lymphocele did not seem to be more accurate than that of general lymphocele. This might be due to the low incidence of complicated lymphocele in our study. However, Benedetti-Panici et al. [13] also reported that there was no significant association of lymphocyst infection with other risk factors including tumor origin, type of hysterectomy, or region of lymphadenectomy. In the same study, all lymphocele with a diameter <5 cm were asymptomatic, whereas all but 2 lymphocysts with a diameter of >5 cm were symptomatic [13]. Mean diameter of lymphocele of our study subjects was 5.6±3.7 cm. Complicated lymphocele was significantly larger than non-complicated ones (mean diameter, 9.5±3.5 vs. 3.8±2.2 cm; P<0.001) (data not shown).
Our study has a few limitations. First, the study population was retrospectively collected from a single tertiary center, which might be the cause of missing values. Second, everyone had surgical drain and the possible protective effect on lymphocele development, if any, could not be evaluated in this study. Lastly, the prediction model requires further external validation before being adopted for general use.
On the basis of readily obtained clinical variables, we developed a nomogram to predict risk of lymphocele in gynecological cancer patients. And predictive accuracy of the model was 0.754. This accuracy is not excellent, however, definitely better than chance. Thus, predicted risk of lymphocele in this nomogram could be used for patient counseling. Surgeons could try to reduce the risk of lymphocele development by minimizing total number of retrieved LNs during surgery, for example, using sentinel LN mapping technique if indicated. In addition, based on the result of great contribution of open surgery to high risk of lymphocele, surgeons might do his or her best not to convert from laparoscopic operation into laparotomy even if it takes longer. Postoperative use of IPC could also be one of the effective strategies for lowering the nomogram score and preventing postoperative lymphocele development. Although the nomogram itself cannot replace clinical observation, however, nomogram not only shows priority of predictors simply through scale's interval but also expresses the probability with user-friendly number.
In conclusion, we developed a nomogram to predict risk of lymphocele in gynecologic cancer patients on the basis of readily obtained clinical variables. It could be helpful for patient consultation and treatment decision making. External validation of this nomogram in different group of patients is needed for gaining popularity in daily routine practice.
Figures and Tables
 | Fig. 1Nomogram for the risk of development of postoperative lymphocele in gynecologic cancer patients. IPC, intermittent pneumatic compression; LN, lymph node; PLN, pelvic lymph node. |
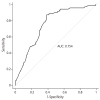 | Fig. 2Receiver operating characteristic curve assessing the predictive accuracy of the nomogram. AUC, area under the curve. |
Table 1
Patient characteristics and their associations with post-lymphadenectomy lymphocele (n=371)

Table 2
Association of post-lymphadenectomy complicated lymphocele with clinicopathologic factors

Table 3
Univariate and multivariate analyses of risk factors for lymphocele

References
1. Hiramatsu K, Kobayashi E, Ueda Y, Egawa-Takata T, Matsuzaki S, Kimura T, et al. Optimal timing for drainage of infected lymphocysts after lymphadenectomy for gynecologic cancer. Int J Gynecol Cancer. 2015; 25:337–341.
2. Gallotta V, Fanfani F, Rossitto C, Vizzielli G, Testa A, Scambia G, et al. A randomized study comparing the use of the Ligaclip with bipolar energy to prevent lymphocele during laparoscopic pelvic lymphadenectomy for gynecologic cancer. Am J Obstet Gynecol. 2010; 203:483.
3. Kim YH, Shin HJ, Ju W, Kim SC. Prevention of lymphocele by using gelatin-thrombin matrix as a tissue sealant after pelvic lymphadenectomy in patients with gynecologic cancers: a prospective randomized controlled study. J Gynecol Oncol. 2017; 28:e37.
4. Kim HY, Kim JW, Kim SH, Kim YT, Kim JH. An analysis of the risk factors and management of lymphocele after pelvic lymphadenectomy in patients with gynecologic malignancies. Cancer Res Treat. 2004; 36:377–383.
5. Charoenkwan K, Kietpeerakool C. Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy for the prevention of lymphocyst formation in patients with gynaecological malignancies. Cochrane Database Syst Rev. 2014; (6):CD007387.
6. Mori N. Clinical and experimental studies on the socalled lymphocyst which develops after radical hysterectomy in cancer of the uterine cervix. J Jpn Obstet Gynecol Soc. 1955; 2:178–203.
7. Metcalf KS, Peel KR. Lymphocele. Ann R Coll Surg Engl. 1993; 75:387–392.
8. Suzuki M, Ohwada M, Sato I. Pelvic lymphocysts following retroperitoneal lymphadenectomy: retroperitoneal partial “no-closure” for ovarian and endometrial cancers. J Surg Oncol. 1998; 68:149–152.
9. Kim JK, Jeong YY, Kim YH, Kim YC, Kang HK, Choi HS. Postoperative pelvic lymphocele: treatment with simple percutaneous catheter drainage. Radiology. 1999; 212:390–394.
10. Park NY, Seong WJ, Chong GO, Hong DG, Cho YL, Park IS, et al. The effect of nonperitonization and laparoscopic lymphadenectomy for minimizing the incidence of lymphocyst formation after radical hysterectomy for cervical cancer. Int J Gynecol Cancer. 2010; 20:443–448.
11. Achouri A, Huchon C, Bats AS, Bensaid C, Nos C, Lécuru F. Complications of lymphadenectomy for gynecologic cancer. Eur J Surg Oncol. 2013; 39:81–86.
12. Tam KF, Lam KW, Chan KK, Ngan HY. Natural history of pelvic lymphocysts as observed by ultrasonography after bilateral pelvic lymphadenectomy. Ultrasound Obstet Gynecol. 2008; 32:87–90.
13. Benedetti-Panici P, Maneschi F, Cutillo G, D’Andrea G, di Palumbo VS, Conte M, et al. A randomized study comparing retroperitoneal drainage with no drainage after lymphadenectomy in gynecologic malignancies. Gynecol Oncol. 1997; 65:478–482.
Supplementary materials
Supplementary table and figure associated with this article can be found online at https://doi.org/10.5468/ogs.2017.60.5.440.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download