INTRODUCTION
Today, resin composites are widely used to build up anterior and posterior dental restorations due to their improved physical-mechanical properties and increasing aesthetic demands [
1]. Resin composites comprise a polymeric matrix, filler particles, and a silane coupling agent that links the matrix to the fillers, as well as minor additional components including initiators, stabilizers, and coloring pigments. As with other composite structures, the type and composition of the resin matrix and the filler particles influence the mechanical properties of these materials, which eventually determine their clinical performance. Resin composite restorations are subjected to temperature variations in the oral cavity induced by diet and to complex chewing forces with a considerable amount of flexural stresses [
12].
When a polymer-based material such as resin is applied in the oral environment, it may incompletely withstand degradation [
34]. As a result, there may be a decrease in its functional properties, such as flexural strength and hardness. Different periods of water storage and thermocycling are among the several methods used to simulate the aging process of dental materials in
in vitro studies. These methods attempt to simulate the hydrolytic degradation that occurs in resin-based composite (RBC) restorations in service [
5]. Many studies have investigated the mechanical properties and degree of conversion (DC) of dental resin composites [
6789]. Dental resin composites used for coronal reconstruction are exposed to moisture and temperature variations. Few of these studies, however, have analyzed the behavior of aged materials. Indeed, the effect of thermocycling on composites can be more detrimental than that of water alone, because of increased degradation of the polymeric network by hydrothermal aging [
10].
It has been found that the degree of monomer conversion in resin composites greatly affects the physical properties of these restorative materials. The DC can be defined as the percentage of reacted carbon-carbon double (C=C) bonds, and can affect many properties of the resin materials, including mechanical properties, dimensional stability, solubility, biocompatibility, and stain susceptibility. The unreacted double bonds may be found either in free monomers or in pendant groups existing on the network. The unreacted monomer may leach from the polymerized dental composites and affect the soft tissues [
11]. The functional performance of a dental resin composite depends on its mechanical, chemical, and biological properties. These properties are related to the polymer network and polymerization parameters, such as the rate of polymerization and DC. However, few studies have examined the DC of aged composites [
2912]. Among the various methods used to determine the DC of resin composites, Fourier transform infrared spectroscopy (FTIR) has been proven to be an effective and reliable tool that works by detecting stretching vibrations of C=C bonds before and after the curing of materials [
13].
Resin composite restorations are exposed to complex chewing forces with a considerable amount of flexural stresses in the oral environment. The flexural strength of a material is the maximum stress that it can resist before failure when subjected to a bending load. The clinical applications determine how much flexural strength is required. For restorations under strong chewing forces, high flexural strength is clearly desired [
14]. Although some published studies have investigated the behavior of aged composite in regard to its ability to bond with new composite or resin luting cement, there is little information in the literature on the physical and mechanical behavior of aged composite itself, such as the DC, microhardness, and flexural strength and how such parameters are related to different degrees of aging.
The objective of this study was to evaluate the effect of thermocycling on the DC, flexural strength, and microhardness of a dental resin composite. Thus, the null hypothesis tested was that thermocycling would not affect the DC, microhardness, and flexural strength of the dental resin composite.
MATERIALS AND METHODS
The dental resin composite used in this study was a microhybrid Filtek Z250 (Shade A1, 3M ESPE, St. Paul, MN, USA). The composition of the material is shown in
Table 1.
Table 1
Composition of organic matrix and filler of Filtek Z250 microhybrid universal dental composite

|
Organic matrix |
Filler |
Manufacturer |
Lot No. |
|
Type |
Vol (%) |
Size (µm) |
|
Bisphenol A diglycidyl ether dimethacrylate (BisGMA), Urethane dimethacrylate (UDMA), Bisphenol A polyethylene glycol diether dimethacrylate (BisEMA), Camphoroquinone (initiator) |
Zirconia/silica |
60 |
0.01–3.5 µm with average of 0.6 µm |
3M ESPE (St. Paul, MN, USA) |
N569982 |

Sample preparation for DC testing
The specimen disks of the dental composite for determining the DC were prepared using a polyethylene mold (7 mm in diameter and 1 mm in height). A small Mylar strip with a thickness of 0.7 mm was placed on a glass slab. The mold was placed on the strip and, after filling the mold to excess, the surface of the material was covered with another Mylar strip and a glass slide, and a weight of 500 g was placed on it for 20 seconds to ensure better accommodation of the resin composite and to remove the excess material. All the composite specimens (n = 5) were light-activated with an LED curing unit (Woodpecker WM-199, Foshan Vimel Dental Equipment Co., Ltd., Guangdong, China) with a light intensity of 500 mW/cm2 and a tip diameter of 7 mm for 20 seconds on both the top and bottom sides. Specimens were then stored in distilled water at 37°C for 24 hours to ensure polymerization. After 24 hours, the specimens were removed from the molds. The specimens were placed in a basket and alternated between 5°C and 55°C water baths with a dwell time of 20 seconds and a rest time of 20 seconds (Dorsa apparatus, Tehran, Iran).
DC testing
The DC was measured in the polymerized specimens. The initial DC (%) of the discs was recorded after 24 hours of storage in distilled water in the absorbance mode using attenuated total reflectance-Fourier transforming infrared spectroscopy (ATR-FTIR; NICOLET iS10, Thermo Fisher Scientific, Waltham, MA, USA) with a Smart OMNI-Transmission accessory. Subsequent measurements of the DC of the thermocycled specimens were made after aging. The DC was measured 7 times in this study: after 24 hours of storage in distilled water, and then after 1,000, 2,000, 4,000, 6,000, 8,000, and 10,000 cycles. Specimens were marked to be examined for DC after the corresponding number of cycles.
The absorption spectra of the cured and uncured composites were obtained at the top surfaces in the region of 400–4,000 cm
−1 wavelength using 4 scans at a resolution of 16 cm
−1. The range from 1,590 to 1,660 cm
−1 was then expanded (
Figure 1). The percentage of unreacted C=C bonds (%) for the methacrylate-based composite was calculated from the ratio of the absorbance peak area of aliphatic C=C bonds (1,638 cm
−1 wavelength) against an internal standard (aromatic C=C, 1,608 cm
−1 wavelength) before and after curing of the specimen using Omnic software. The DC was then calculated by subtracting the percentage of the C=C bonds from 100%, according to the following equation.
 | Figure 1Fourier transform infrared spectroscopy (FTIR) spectra of the resin composites before (upper red) and after setting (lower green).
|
The mean values of DC after a certain number of thermocycles were compared using 1-way analysis of variance (ANOVA). The Tukey honest significant difference (HSD) test was used for pair-wise comparisons. All statistical analyses were performed at a significance level of α = 0.05.
Sample preparation for microhardness test
The specimens were fabricated using circular polyethylene molds (7 mm in diameter and 1 mm in width) for the Vickers microhardness test. Specimens were light cured with an LED curing unit and stored in a water bath at 37°C for 24 hours. Then, the specimens were randomly divided into 4 groups (n = 10). The surface microhardness of the specimens was measured using a microhardness tester (Baresiss, Oberdischingen, Germany) under a load of 300 g for 15 seconds for 10 specimens (G1). The average value of the 3 indentations for each specimen was taken as the Vickers hardness number (VHN). The remaining specimens were subjected to thermocycling using 5°C and 55°C water baths, with a dwell time of 20 seconds. Microhardness was assessed again after 1,000, 4,000, and 10,000 cycles (G2–G4). Data were analyzed using 1-way ANOVA and the Tukey HSD post hoc test.
Sample preparation for flexural strength test
For the flexural strength test, a stainless-steel mold (25 × 2 × 2 mm) was utilized to prepare the specimens according to ISO 4049. The mold was placed on a glass slide over a Mylar sheet. Light-cured resin composite was condensed into the mold, and a Mylar sheet and another glass slide were placed over them. The assembly was placed under a clamp and adequate pressure was applied. The middle third of the specimen was first cured for 20 seconds with a 650 mW/cm2 LED, and then the remaining thirds were cured in the same manner as the middle third by overlapping with the central part. The same procedure was applied to the lower surface of the specimen. After irradiation, the assembly was placed into a water bath at 37°C for 15 minutes. The flash was then removed with the use of sandpaper, and the test specimens were separated from their molds and again stored in a water bath at 37°C for 24 hours.
Twenty-four hours after irradiation, the specimens were randomly divided into 4 groups (n = 10). Flexural strength testing was then carried out with a universal testing machine (Santam 20, Santam Design & Manufacturing Company, Tehran, Iran) with a load cell of 6 kg (Bongshin, Seongnam, Korea) at a crosshead speed of 0.5 mm/min until the specimen was fractured. The maximum load exerted on the specimen was recorded, and flexural strength (in MPa) was calculated with the following equation.
The same procedures were followed after 1,000, 4,000, and 10,000 cycles. Data were analyzed using 1-way ANOVA and the Tukey HSD post hoc test.
Scanning electron microscopy (SEM) analysis
In order to investigate the effect of thermocycling on the surface degradation of resin composites, 2 specimen discs were analyzed by SEM. The discs were dried, sputter-coated, and observed using SEM (TESCAN, VEGA, Brno, Czech Republic). The SEM images were taken at ×2,000, ×5,000, and ×15,000 magnifications.
DISCUSSION
Aging of RBCs is often the outcome of mechanical mechanisms of physical degradation such as wear, abrasion, and fatigue. It may also be due to mechanisms of chemical degradation such as temperature-related breakdown or enzymatic, hydrolytic, and acidic processes. Exposing the specimens to thermocycling regimens is a common technique for simulating hydrothermal aging [
515]. Alternating temperatures between 5°C and 55°C, coupled with the presence of water, contributed to the aging of RBC in this study. Our objective was to investigate artificial aging-induced changes in microhybrid dental composites. Specimens were subjected to thermal aging ranging from 1,000 to 10,000 cycles in the DC test. According to ISO 11405, the use of 500 thermal cycles between 5°C and 55°C is considered to be suitable to simulate short-term aging of dental materials [
16]. In addition, Gale and Darvell [
17] proposed that 10,000 cycles might represent approximately 1 year of
in vivo functioning, with 20 to 50 cycles considered equivalent to a single day. Therefore, we decided to continue thermal cycling to 10,000 cycles to examine the results.
ATR-FTIR was employed in the absorbance mode in this study to determine the DC of the dental resin composite. This method is reliable and is not time-consuming. This technique is non-destructive, requires no sample preparation, and does not involve pulverization. Consequently, we were able to measure the DC at different times without losing the samples. The DC measurements increased up to 4,000 cycles. These findings are in accordance with the study of Pallikari and Iosifidou [
12], who reported further advances in the DC of resin composites after artificial aging. However, in the aforementioned study, the aging of the samples was performed in 2 steps — initially by thermocycling and then by UV irradiation — and corresponded to 5 years of natural aging [
12]. In a study conducted by Tonetto
et al. [
8], the DC of resin cements was investigated at 24-hour intervals until 7 days after light curing in the dry state in a dark room. They found the greatest DC at 7 days after curing, with completion of the polymerization reaction [
8].
There are 2 possible explanations for why the DC increases over time. First, the elution of unreacted monomers caused by the release of monomers from the dental resin composite increases with thermal shocks and storage time [
18]. Our study showed that the storage of specimens in water without thermal cycling did not have a considerable effect on DC. Therefore, it can be concluded that the increased temperature during thermal cycling may accelerate monomer dilution by increasing monomer diffusion. Sideridou and Achilias [
19] detected leached monomers of this composite (Filtek Z250) using high-performance liquid chromatography. More of the monomer urethane dimethacrylate leached than other monomers, although a small amount of triethylene glycol dimethacrylate (TEGDMA) was detected. TEGDMA, which is a hydrophilic monomer, has been found to be released faster than other monomers [
19]. In fact, several factors affect the leaching of monomers, including the type of monomers and fillers, the chemical structure of the resin, the resin/filler proportion, the type of the curing unit, and the elution medium [
18]. Durner
et al. [
20] measured the DC and the amount of elutable substances from resin composites as a function of polymerization time (5–40 seconds). They observed a significant increase in DC as the polymerization time increased from 5 to 40 seconds, whereas the amount of elutable substances decreased, except for methacrylic acid [
20].
The second reason may be continuation of the polymerization reaction of entrapped unreacted monomers. According to Andrzejewska [
21], some free radicals entrapped in the polymer during the vitrification stage of dental resin composites may remain in the network for several weeks after vitrification [
2122]. Studies suggest that increasing temperature or swelling by the solvent or diffusion reaction can increase the mobility of the system, making such radicals able to react with any remaining double bonds within the network and continue the polymerization process [
23]. In addition to free radicals, there are other ‘trapped’ active components in the polymer network, such as free monomers, pendant double bonds, and photoinitiators [
24]. It has been reported that heating of the composite resin to a temperature of 55°C (close to the glass transition temperature [Tg]) is probably the driving force for continuing the polymerization. According to Truffier-Boutry
et al. [
25], the Tg of an organic matrix such as composite resin is close to 55°C. Storing the specimens at high temperatures close to the Tg could enhance the molecular mobility in the polymer matrix, causing radical recombination to occur. At temperatures below the Tg, the molecular mobility is low because the network is in a vitrified state [
25]. Moreover, increasing the temperature may promote a diffusion reaction, so that small molecules can diffuse into the polymer matrix and encourage radical termination [
22].
In this study, the DC of C=C double bonds in our composite was 60% ± 3.3% at the baseline, suggesting that approximately 40% of the C=C double bonds were ‘uncured.’ Leaching of uncured monomer, as mentioned before, may decrease the ratio of aliphatic to aromatic C=C bonds in the aged state compared with the cured state at the baseline. Subtracting this diminished proportion of C=C double bonds from 100%, according to the formula, may increase the amount of DC, which is in agreement with what we observed in our study. The spectra taken within the period of the experiment exhibited similar peaks/bands. However, the changes in C=C related peak areas indicated an enhanced DC. Furthermore, on FTIR spectra, the band in the 3,100–3,700 cm−1 range tended to be stronger with increasing aging time. This strong band can be interpreted as the O-H stretching band derived from increasing amounts of absorbed water over time.
ISO 4049 proposed a 3-point bending method for evaluating flexural strength [
26]. Furthermore, in most articles, 3-point bending has been employed for the assessment of dental resin composites. Accordingly, we chose this method to allow a good comparison with previous studies. The measured values of flexural strength (117.04 ± 21.72 MPa) obtained from the microhybrid dental composite tested in this study without thermocycling was similar to those observed in other studies of Z250 composites. Attar
et al. [
27] found that the flexural strength was 117.4 ± 19.2 MPa at 24 hours and 95.6 ± 10.6 MPa after 1 month, and Chung
et al. [
6] found values ranging from 66.61 to 147.21 MPa after 1 week of storage in water. In fact, composite resins have a brittle nature and with increased aging time, they become more brittle, as shown by the superficial changes, degradation of the resin matrix, and debonding of fillers, which are clearly observed on SEM. Thus, we observed a significant decrease in flexural strength values after thermocycling, in good agreement with the findings reported by Souza
et al. [
2] and Janda
et al. [
28]. Therefore, the null hypothesis that thermal aging has no effect on the flexural strength of RBCs should be rejected. High or elevated temperatures are also known to weaken the resin-based materials. A minimum flexural strength of 80 MPa is considered appropriate by the ISO for restorative materials involving outer occlusal surfaces [
26]. The results of the present study of up to 1,000 hydrothermal cycles exceeded the value required by ISO 4049 (80 MPa), but the flexural strength values after 4,000 cycles were below 80 MPa, and thus did not meet the limits of ISO 4049 for use as occlusal fillings.
The SEM micrographs produced at the end of the experiments in this study revealed superficial deterioration and a softer surface (
Figure 3). Thus, we expected lower hardness values. With regard to the obtained microhardness results, there was a decreasing trend with continuing the thermal cycling of the specimens. In agreement with our study, de Moraes
et al. [
29] presented a significant decrease in hardness values and softer surfaces of a Z250 composite after 6 months of storage in water. For the subsurface, a decrease in hardness values after 1 year was reported [
29]. Studies such as those conducted by Pereira
et al. [
30], and Souza
et al. [
2] in indirect resin composites (IRCs) have investigated the effect of thermocycling on microhardness. Pereira
et al. [
30] reported a significant decrease in microhardness values of 2 tested materials after 3,000 cycles of thermal cycling. However, Souza
et al. [
2] found that 5,000 thermal cycles did not decrease the microhardness of most IRCs. de Oliveira
et al. [
15] reported that the hardness of the microhybrid resin composite (Opallis) did not change either after storage in water or after thermocycling. It has been reported that polymeric chains with a high crosslinking density showed less water absorption due to the reduction of the free space. Moreover, in materials with a denser polymer network, less water absorption is expected [
15]. Ferracane and Condon [
31] reported a rapid elution of unreacted molecules and much slower water sorption after soaking of resin composites in water, in a process controlled by diffusion into the polymer matrix. During this gradual water sorption, water molecules tend to occupy the free volume between the cross-linked chains and micro-voids created during polymerization [
32]. Slow water uptake of resin composite corresponds to the results of our study, in which significant alterations were found after 4,000 cycles in all 3 tests.
 | Figure 3 Scanning electron microscopy (SEM) micrograph of the Filtek Z250 resin composite at ×15,000 magnification. (A) Freshly cured material; (B) After 10,000 cycles of thermocycling; (C) After 14,000 cycles of thermocycling. Coarse protruding filler particles were present in the weakened resin matrix at the thermocycled resin composite surface. Some areas from which filler particles seem to have been debonded and plucked out were observed. The composite showed exposed particles and loosening of filler particles after aging.
|
The analysis of SEM images after aging has revealed deterioration in RBC surfaces. Hydrolytic degradation, as discussed above, can be explained by the fact that some electropositive ions that exist in filler particles tend to react with water, changing the internal charge balance of the silica matrix. By increasing the number of hydrogen ions that occupy the free spaces, the (Si-O-Si) bonds of the silica matrix break, and surface degradation and superficial softening thus occur with aging [
3]. Prolonged thermal cycling can lead to water absorption and swelling of the matrix. Water sorption is considered a diffusion-controlled process in composite resins. The water taken up by the polymer network could result in filler-matrix debonding, softening of resins, and even the hydrolytic degradation of fillers. This could affect the mechanical properties of resin composites. In fact, the role of water is like a plasticizer that could weaken the polymer network. It can directly degrade the matrix-filler interface by causing the breakdown of chemical bonds of the silane-filler interface and filler particle surfaces. Unfortunately, the minimal number of thermal cycles required for complete plasticization is not known [
24].
It seems that with the leaching of unreacted monomers over time, the findings become more homogeneous and show less variation. The observation of a smaller standard deviation and CV in specimens at the end of thermocycling protocol, particularly in the DC test, shows the higher predictability of the DC data in samples subjected to aging.
Although this study was conducted to simulate aging by thermocycling, resin composite materials in clinical situations are also subjected to the effects of beverages, food, mouthwashes, saliva (pH, composition, etc.), and the mechanical actions of chewing and brushing. Thus, these factors should be considered in future research. The performance of dental resin composites in clinically realistic circumstances needs further investigation. Despite the deteriorative changes that occurred in the current study, such considerations should be taken into account for composites subjected to aging. Due to financial limitations and the large amount of material used for tests in this study, only one material was tested. Within the limitations of this study, we evaluated a microhybrid resin composite to gain initial insights into the properties of this material. The mechanical properties and DC of other types of resin composites remain to be investigated in future studies.

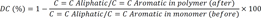








 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download