INTRODUCTION
Choledochal cysts are rare congenital malformations of the bile duct characterized by abnormal dilatations of the intrahepatic and/or extrahepatic portion of the biliary tree.
1 The worldwide prevalence of choledochal cyst ranges from 1:13,000 to 1:2,000,000,
2 with majority of cases reported in Southeast Asian countries (e.g., Japan), where the prevalence could be as high as 1:1,000, when compared to Western countries where the prevalence is 1:100,000 to 150,000. Also, choledochal cyst predominantly affects females (3-4:1). A notable observation is that Asians immigrants to Western countries have reported lesser prevalence of choledochal cyst than in their native countries.
3
From the anatomical perspective, choledochal cyst is associated with an anomalous arrangement of the pancreaticobiliary duct.
1 Therefore, patients have a higher lifetime risk for developing acute pancreatitis, cholangitis and biliary tract malignancy (e.g., cholangiocarcinoma).
1234 Nonetheless, patients with choledochal cyst have an excellent prognosis with early diagnosis and surgical resection with reconstruction, before life-threatening complications arise. On the other hand, pancreas divisum is the most frequent congenital pancreatic variation; this anomaly results from a fusion failure of the ventral and dorsal pancreatic buds.
5
The coexistence of choledochal cyst and pancreas divisum in adults is extremely rare. This article aims to present the first case of a Latin America patient with choledochal cyst and pancreas divisum. In addition, a literature review and discussion of the clinical features of this entity is provided.
Go to :

CASE
A 42-year-old Peruvian man hospitalized in Peru, with recurrent abdominal pain and diagnosis of chronic lithiasic cholecystitis, underwent open cholecystectomy. During the surgical procedure, choledochal cyst and a partial obstruction of the choledochal duct was found. Thus, a hepaticoduodenostomy was performed without removing the cyst. No complications were reported during or after the surgery.
Seven months after the surgical procedure, he presented with recurrent abdominal pain with belt-like radiation, which was exacerbated with cholecystokinetics, and associated with chronic diarrhea and steatorrhea. He was then referred to our hospital. At admission, his laboratory tests revealed elevated serum amylase (299 U/L) and serum lipase (597 U/L), with remaining liver function tests within normal range. This led to the diagnosis of chronic pancreatitis. During subsequent endoscopic retrograde cholangiopancreatography (ERCP) procedure, pancreas divisum and amputation of the major pancreatic duct (Wirsung) was found (
Fig. 1). To confirm the diagnosis, the patient underwent an abdominal computed tomography scan, where a diverticular formation on the pancreatic head was reported. The patient then underwent an exploratory laparotomy, with the operative finding of pancreas divisum, congenital choledochal cyst of 20 mm in diameter and hypoplasia of the distal part of the major pancreatic duct and the major duodenal papilla, which emptied into the hypoplastic portion with flow disruption of the ventral pancreas. With histopathology findings of a major duodenal papilla with chronic inflammation and fibrosis with chronic nonspecific duodenal inflammation, a transduodenal papillectomy was performed. The postoperative course was uneventful.
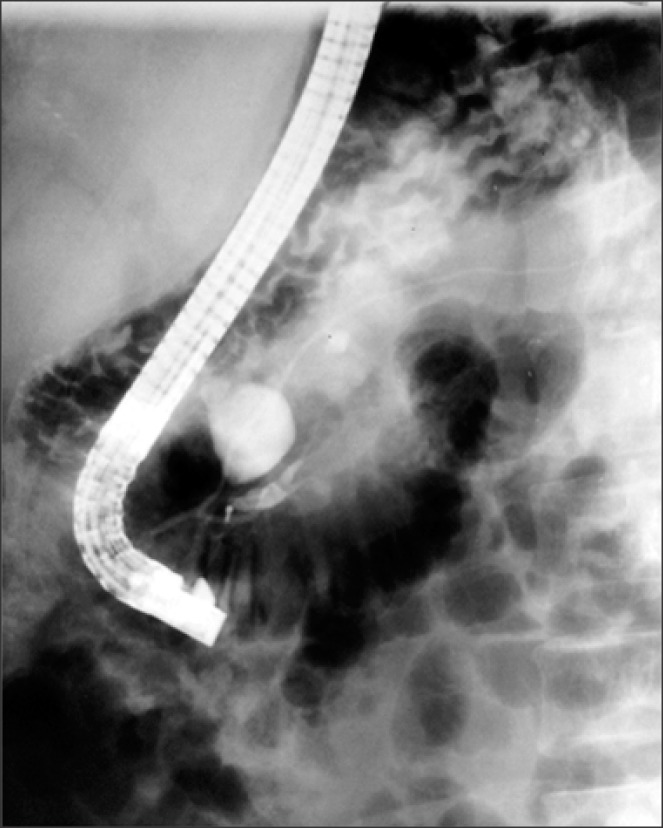 | Fig. 1Endoscopic retrograde cholangiopancreatogram. Injection of iodine contrast agent into the major duodenal papilla reveals its amputation. Opacification of the principal and secondary pancreatic duct with an independent drainage is identified. A saccular image was also observed on the common bile duct.
|
The patient again presented abdominal pain 8 years later, having the same clinical characteristics associated with fever, asthenia, adynamia and involuntary weight loss of 5 kg since the last 3 months. Laboratory studies showed elevated serum amylase and lipase, diagnosing recurrent acute pancreatitis with associated cholangitis. The patient was hospitalized, seven days later he was discharged without complications with normal serum lipase levels, but persistent elevated amylase levels (152 U/L).
However, 7 years later he presented acute cholangitis with following laboratory reports: total bilirubin, direct bilirubin and indirect bilirubin within normal levels, and elevated levels of alkaline phosphatase 171 U/L, aspartate aminotransferase 69 U/L and alanine aminotransferase 67 U/L. The patient then underwent a percutaneous transhepatic cholangiography (PTC), where a hepaticoduodenostomy stenosis was identified. Subsequently, a balloon dilatation and placement of an internal-external biliary catheter was performed. Next, he underwent choledochal cyst resection and dismantling of the hepaticoduodenostomy with Roux-en-Y hepaticojejunostomy, with partial resection of the IV B hepatic segment. The patient was discharged 5 days later, asymptomatic and without any complications.
The patient remained asymptomatic for 12 years, after which he presented with acute cholangitis. A percutaneous biliary drainage was performed, revealing a leak to the abdominal cavity. Subsequently, a magnetic resonance imaging showed loss of proximal left branch continuity, a T1-hypointense pericholangitic lesion consistent with inflammatory activity, as well as pneumobilia on the left hepatic lobule, leading to the diagnosis of left hepatic duct stenosis. Subsequently, the patient underwent a hepatojejunostomy of the left duct. Four days after surgery, the patient was discharged with all laboratory levels within normal limits. Currently, the patient remains asymptomatic 18 months after the surgery.
Go to :

DISCUSSION
Choledochal cyst association with pancreas divisum in adults is a rare condition, and in our knowledge less than 8 well-documented cases have been reported.
256789 In this patient, the choledochal cysts was categorized as type I according to the Todani et al. classification, due to the cyst localization on the extrahepatic biliary tree (
Table 1). This variant is the most frequent choledochal cyst type, while pancreas divisum is the most common congenital pancreatic variant, being found in 10% of necropsies.
10 In the latter cases, the ventral duct drains the ventral pancreas through the major papilla and the rest of the pancreatic tissue drains via the dorsal duct through the minor papilla.
5 When a choledochal cyst type I coexists with a sufficiently wide common pancreatic duct, the bile and pancreatic juice drainage may not have any repercussions. However, if the duct is narrow, there is accumulation of fluids, and pancreatitis with secondary cholangitis can be presented. In a recent study, Bertin et al.
11 demonstrated that patients with pancreas divisum are more likely to have genetic predisposition to chronic pancreatitis involving defects in
CFTR,
SPINK1, and
PRSS1. Choledochal cyst increases the risk of developing cholangitis, acute pancreatitis and cholangiocarcinoma, and hence cyst excision is recommended. Nevertheless, successful liver transplants have been performed in cases of bile duct malignancies secondary to choledochal cyst.
31213 A delayed diagnosis can result in biliary cirrhosis and portal hypertension, which is the most common long-term complication of this pathology.
14
Table 1
Todani classification for choledochal cyst15
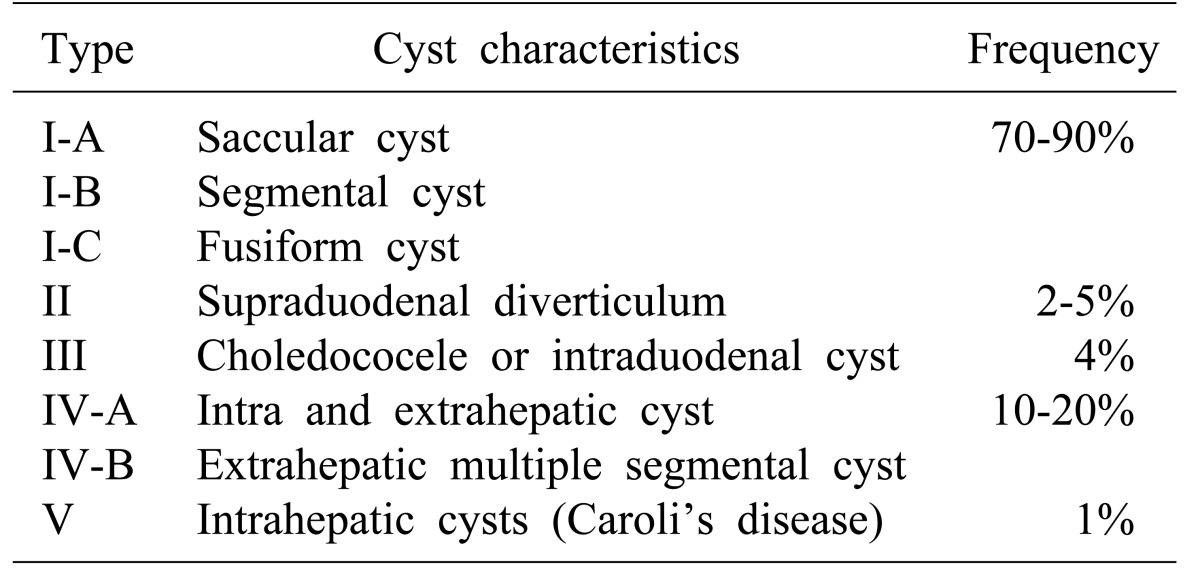

According to Todani et al.,
15 the most common clinical presentation of choledochal cyst is intermittent recurrent episodes of upper abdominal pain. The classic triad of the choledochal cyst includes abdominal pain, intermittent jaundice and palpable right upper quadrant abdominal mass; however, these are found in the minority of cases.
16 Patients with this condition usually present acute pancreatitis with hyperamylasemia, as in the current case report. However, if the physician does not suspect choledochal cyst and further evaluation is not done, the diagnosis might be easily overlooked.
ERCP is considered the gold standard for the diagnosis of pancreas divisum due to its high diagnostic accuracy. However, cannulation of the minor papilla is difficult during ERCP, and the technique presents a high rate of complications. Due to these disadvantages, the use of noninvasive procedures, such as magnetic resonance cholangiopancreatography (MRCP), multidetector computed tomography (MDCT) and endoscopic ultrasonography, are well-accepted as diagnostic tools in the assessment of bile tree duct anatomy.
17 MRCP enables a noninvasive diagnosis of pancreas divisum without the use of contrast material, and avoids the risk of ERCP induced acute pancreatitis.
18 MDCT may also identify this anomaly, and its diagnostic accuracy can be improved with the secretin stimulation test.
1718 In a series of 45 patients with pancreas divisum, Kushnir et al.
19 found that endoscopic ultrasonography has more sensitivity for establishing the diagnosis of pancreas divisum, than MDCT or MPCP.
In cases of suspected biliary pathology, the initial imaging study should be abdominal ultrasonography.
16 Nevertheless, ultrasound is less accurate for the diagnosis of bile duct cysts in adults, where the cause of bile duct dilatation may differ (e.g., malignancy or other non-malignant entities).
20 Therefore, for a precise and accurate delimitation of the biliary tree, a cholangiography is necessary.
16 In order to achieve this goal, complementary diagnostic assessments include computed tomography, MRCP and ERCP. Some patients may also present an anomalous pancreaticobiliary junction that can be diagnosed by MRCP or ERCP.
21 MRCP is emerging as a highly sensitive, safe and preoperative noninvasive diagnostic technique for choledochal cyst detection. Hence, in clinical practice, MRCP is recommended before ERCP, in patients suspected of having choledochal cyst on ultrasonography screening.
20 Although choledochal cyst is not a frequent condition, the surgeon should always consider rgeon should always consider the possibility of choledochal cyst during a surgical exploration procedure in patients with biliary tract-related symptoms.
16
Current guidelines recommend that patients with pancreatic-type abdominal pain and pancreas divisum should undergo smoking cessation before considering any endoscopic or surgical intervention, due to an increased risk of postsurgical complications in smokers. The majority of patients with pancreas divisum do not exhibit symptomatic chronic pancreatitis and should not be considered candidates for surgical treatment.
22 ERCP has an important therapeutic role in the endoscopic treatment of patients with symptomatic pancreas divisum. There are no prospective randomized controlled trials comparing endoscopic and surgical therapy; nonetheless, retrospective studies could not establish a difference between the pooled overall response rates between both treatments.
17
Recurrent pancreatitis, cholangitis or malignancy are complications associated with choledochal cyst; hence, an early diagnosis and definitive surgical management needs to be performed. Currently, the total resection of the extrahepatic bile duct with hepaticojejunostomy is the treatment choice for choledochal cyst. Early diagnosis and surgical treatment provide a good prognosis, with few complications in most cases. Although choledochal cyst shows an excellent long-term prognosis with early resection, surgical complications increase with age.
23 Nevertheless, the risk of biliary malignancy remains elevated for 15 years or more after choledochal cyst excision.
24 Therefore, long-term surveillance is essential, especially if there was persistent dilatation of the intrahepatic bile duct or biliary stones.
1 The follow-up should consist of regular abdominal ultrasonography and liver function test evaluation.
2324
Management of choledochal cysts depends on the type of cyst. Type I, II and IV-A treatment consist of complete extrahepatic bile duct cyst excision down to the level of communication with the pancreatic duct, cholecystectomy, and restoration of bilioenteric continuity. Hepaticoduodenostomy and Roux-en-Y hepaticojejunostomy reconstruction after choledochal cyst resection are both reported in literature, but Roux-en-Y hepaticojejunostomy is the preferred technique. Hepaticoduodenostomy is associated with increased rates of gastric cancer, biliary cancer, postoperative reflux and gastritis.
24 Recently, there is a report of a successful cyst excision and Roux-en-Y hepaticojejunostomy via laparoscopy, with faster recovery, less intestinal adhesions and aesthetic damage, and facilitation of surgery due to magnification of the operative field.
23
Hackert et al.
8 reported a similar case that was successfully treated with bile duct resection, papillectomy, hepaticojejunostomy and jejunal reinsertion of the uncinate pancreatic duct. However, there is a lack of evidence about the efficacy and safety of this procedure.
In conclusion, the association of pancreas divisum with choledochal cyst is a rare condition that should be suspected in adult patients with recurrent acute cholangitis and/or pancreatitis, without an identified cause. Additionally, imaging studies should be used for diagnosis, considering that MRCP and endoscopic ultrasonography have a higher sensitivity compared with the current gold standard (ERCP). When choledochal cyst and pancreas divisum coexist, early diagnosis and resection are the main objectives due to the increased risk of cholangiocarcinoma and other complications. In addition, a long follow-up duration should be considered after surgical resection of the cyst. Although pancreas divisum with choledochal cyst could have a favorable prognosis, more studies are required for determining the best therapeutic approach and overall prognosis of this group of patients.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download