Abstract
Purpose
This study aimed to assess the reliability of measurements performed on three-dimensional (3D) virtual models of maxillary defects obtained using cone-beam computed tomography (CBCT) and 3D optical scanning.
Materials and Methods
Mechanical cavities simulating maxillary defects were prepared on the hard palate of nine cadavers. Images were obtained using a CBCT unit at three different fields-of-views (FOVs) and voxel sizes: 1) 60×60 mm FOV, 0.125 mm3 (FOV60); 2) 80×80 mm FOV, 0.160 mm3 (FOV80); and 3) 100×100 mm FOV, 0.250 mm3 (FOV100). Superimposition of the images was performed using software called VRMesh Design. Automated volume measurements were conducted, and differences between surfaces were demonstrated. Silicon impressions obtained from the defects were also scanned with a 3D optical scanner. Virtual models obtained using VRMesh Design were compared with impressions obtained by scanning silicon models. Gold standard volumes of the impression models were then compared with CBCT and 3D scanner measurements. Further, the general linear model was used, and the significance was set to p=0.05.
Results
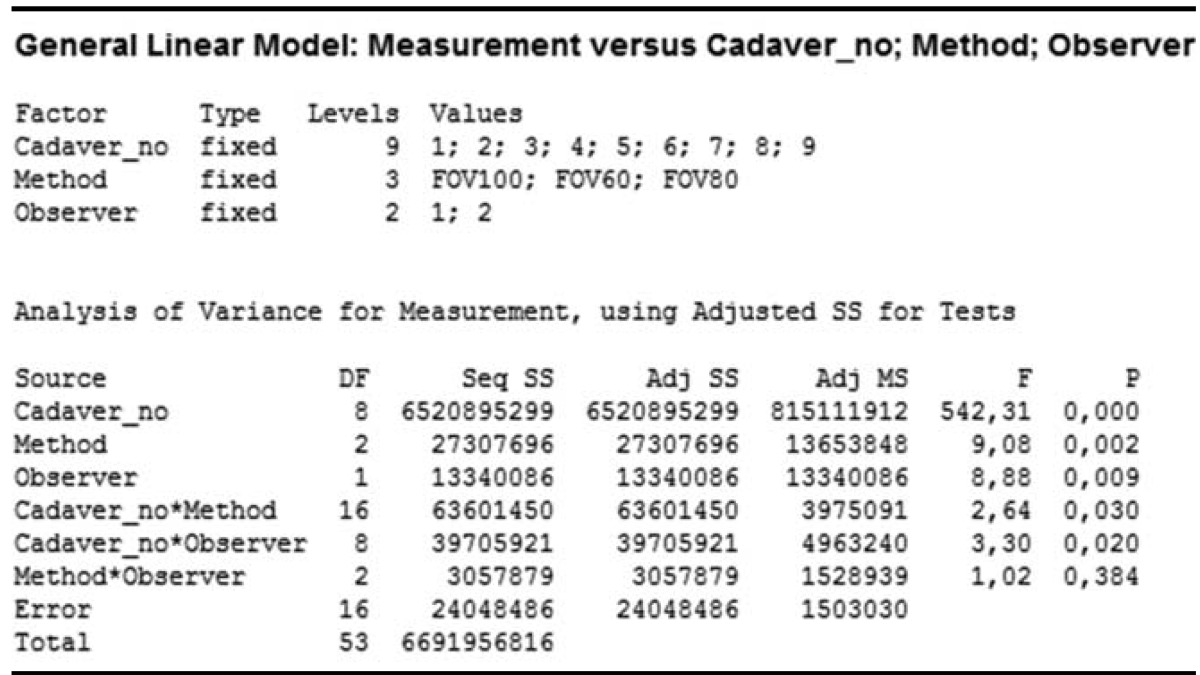
A comparison of the results obtained by the observers and methods revealed the p values to be smaller than 0.05, suggesting that the measurement variations were caused by both methods and observers along with the different cadaver specimens used. Further, the 3D scanner measurements were closer to the gold standard measurements when compared to the CBCT measurements.
Maxillofacial defects as a result of the surgical resection of tumors are usually reconstructed with maxillofacial prosthetics. The fabrication of these prostheses most commonly involves the construction of plaster study models obtained using conventional impression techniques. This is a difficult procedure not only for the patient but also for the prosthodontist who must take accurate impressions of a large defect. In this regard, conventional impression techniques suffer from deformation or tearing of the material when the impression is removed from the mouth and loss of impression accuracy due to nasal mucosal secretion on the impression material.1 Recently, the use of virtual planning for the restoration of tissues that are lost due to trauma or tumor surgery has become popular in the field of reconstructive surgery. In addition, application of computer-aided diagnosis and computer-aided manufacturing facilitates planning and execution, particularly in complex anatomical situations involving different layers of tissues.2
Cone-beam computed tomography (CBCT) uses a cone-shaped X-ray beam centered on a two-dimensional (2D) sensor to scan a 180°-360° rotation around the patient's head in order to acquire the full three-dimensional (3D) volume of data. CBCT systems offer different sensor types, fields of view (FOVs), and exposure settings. Further, CBCT allows 3D imaging of the patient's head and stores the acquired data in a volumetric dataset without superimposition of anatomical structures and magnification.3,4,5,6 The volumetric dataset from the CBCT can be visualized in different ways such as multiplanar reformatted (MPR) slices, panoramic reformatted images, volume-rendered images, and surface-rendered images. CBCT systems are also capable of providing radiographic rather than reflective-based imaging and may be utilized in the construction of the necessary virtual impressions; this is the first step in prosthesis reconstruction. With recent advancements, it has become possible to fuse digital impressions from computer-aided diagnosis and manufacturing systems with a CBCT dataset. Disadvantages associated with dental CBCT include scatter radiation, limited dynamic range, minimal soft-tissue detail, and beam hardening artifacts caused by dental-care materials and implants.3,4,5,6
The aim of the present study was to assess the reliability and validity of measurements performed on 3D virtual models of maxillary defects obtained using CBCT with different FOVs and a 3D optical scanner by comparing them with measurements performed on actual defects created ex vivo in human cadavers. The ultimate goal of this project was to develop a reproducible, reliable, and useful clinical method to create accurate virtual models from which obturating maxillofacial prostheses could be fabricated.
Approval for the use of cadaver heads (n=9) was obtained from Gülhane Military Medical Academy, Department of Anatomy (Local Ethics Committee Review Number 1491-304-12/1539-604). Mechanical cavities of various sizes and dimensions simulating localized maxillary defects were prepared on the palatinal aspect of the hard palate by using round and cylindrical dental burs to their full depth.
Images of the cadavers were obtained using a CMOS flat panel detector, variable FOV CBCT unit (3D Accuitomo 170, J. Morita Mfg. Corp., Kyoto, Japan) operating at 90 kVp and 5.0 mA and having an exposure time of 17.5 s to image each specimen after defect preparation at three different FOVs and voxel sizes (nominal cubic millimeter resolution [mm3]: 1) 60 mm×60 mm FOV, 0.125 mm3 (FOV60); 2) 80 mm×80 mm FOV, 0.160 mm3 (FOV80); and 3) 100 mm×100 mm FOV, 0.250 mm3 (FOV100).
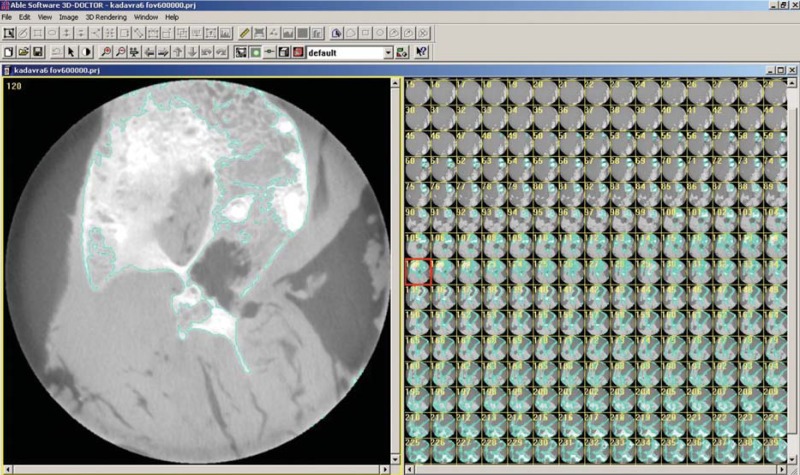
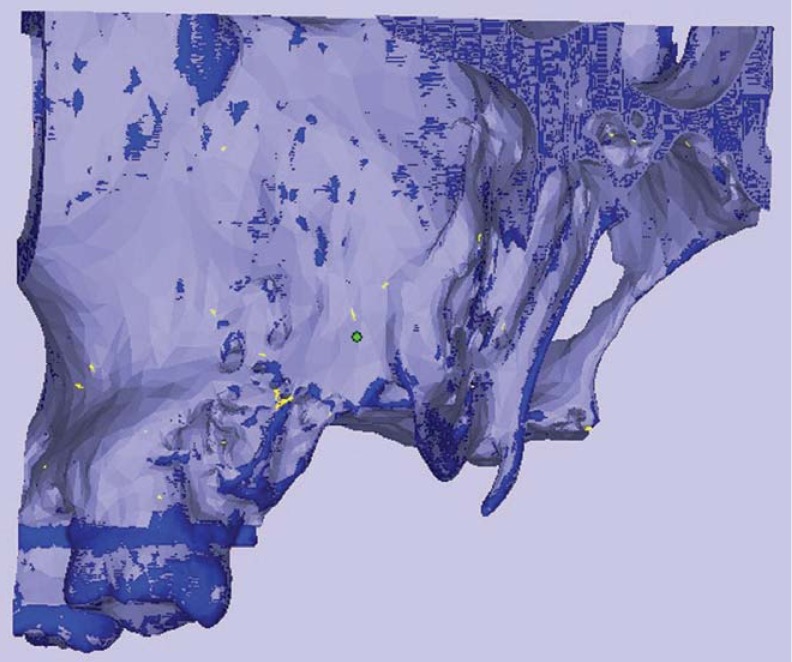
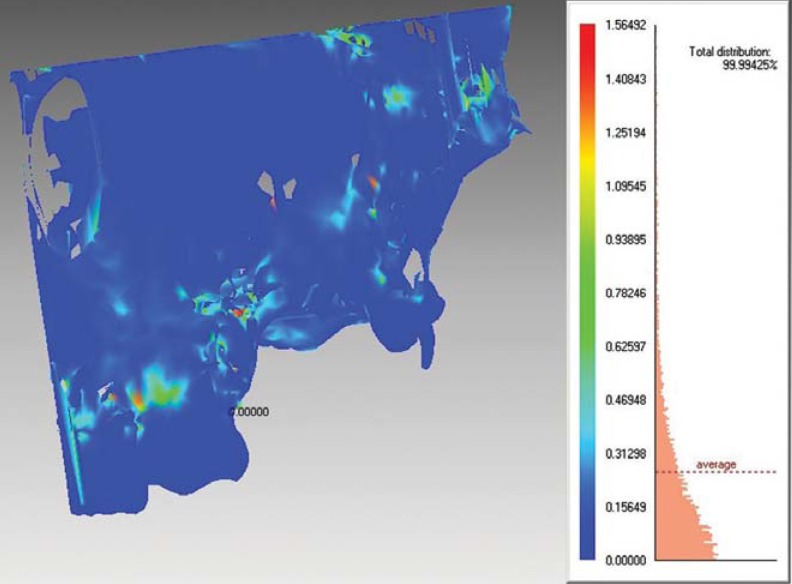
For a comparison of CBCT images taken with different FOVs, axial CBCT scans taken at each acquisition parameter of nine defects were exported as DICOM files and then imported into volumetric rendering software capable of measurements of a vector-based segmentation technology (3D Doctor, Able Software Corp., Lexington, MA, USA). This software allows defect segmentation on consecutive axial slices, enabling defect visualization at each level. This ensured manual, detailed slice-by-slice segmentation of the defect borders by using a mouse with color delineation (turquoise green). The maxillary cadaver images in which defects were identified were outlined with the mouse by using a tool called "Free" to remark at the region of interest. Figure 1 shows the segmentation process with 3D Doctor. After reconstruction with 3D Doctor, superimposition of the maxilla images obtained at different FOVs was performed by using VRMesh Design (Virtual Grid, Bellevue City, WA, USA). Then, virtual models obtained at each FOV were cut from the common intersection points in order to obtain identical borders of the maxillas. Thereafter, automated volume measurements were conducted, and differences between surfaces were demonstrated. Figure 2 shows the alignment of standardized FOV images, and Figure 3 shows the measurement of differences using standardized FOV images.
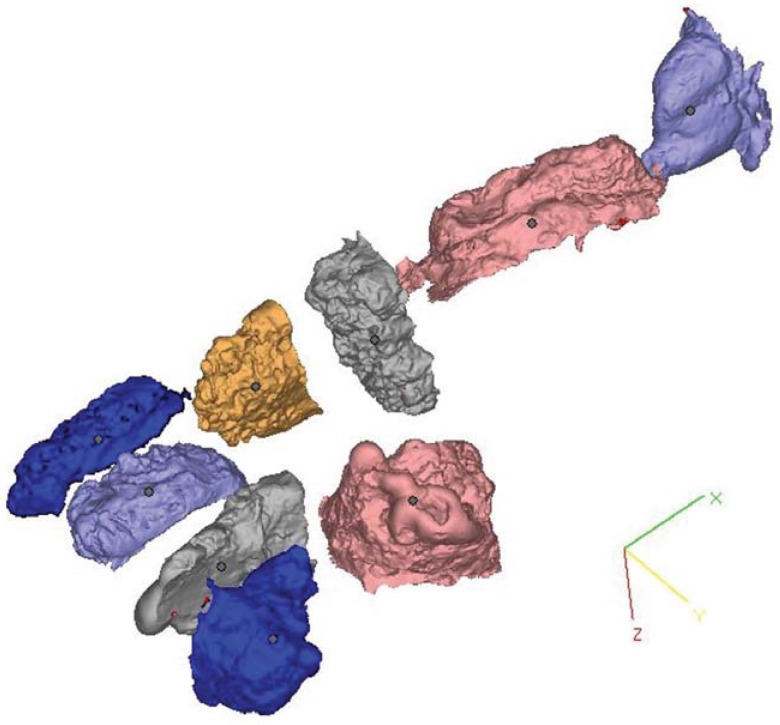
Silicon impressions obtained from the defects were also scanned with a Smart Optics Activity 880 3D Dental Scanner (Smart Optics, Bochum, Germany). Virtual models obtained using VRMesh Design were compared with impressions obtained by scanning silicon models. Virtual models obtained from nine maxillary defects are shown in Figure 4.
In addition, a non-deformable light flow silicon impression material (Variotime; Heraeus-Kulzer, Hanau, Germany) was injected into the defects, and the volumes of the impression models were measured by using the "water displacement technique." This technique can be mathematically expressed as follows: Lost volume of the water in the cylinder - initial volume of the water in the cylinder=defect volume. Volumetric measurements of the impression models were taken twice with a 1-week interval using a Scaltec SBC 21 balance (Denver Instrument, Bohemia, NY, USA) by an external independent researcher (physiologist). The average volume was considered the reference standard. All physical measurements were then compared with the CBCT and 3D optical scanner measurements.
All measurements were performed twice by two observers, and an average was compared to the physical volume calculated from the impression material. The images were viewed in a dimly lit room on a 15.6-in laptop monitor (F75 5-3D350, Qosmio, Toshiba, Tokyo, Japan) at a screen resolution of 1920×1080 and color depth of 32 bits. Each examiner was trained until he/she felt comfortable with the use of the software tools. The gold standard results were used to validate the accuracy of the CBCT and 3D optic scanner readings in the assessment of the defect volume and to compare any difference between these findings. Further, the general linear model was used as the statistical analysis method, and the significance was set to p=0.05.
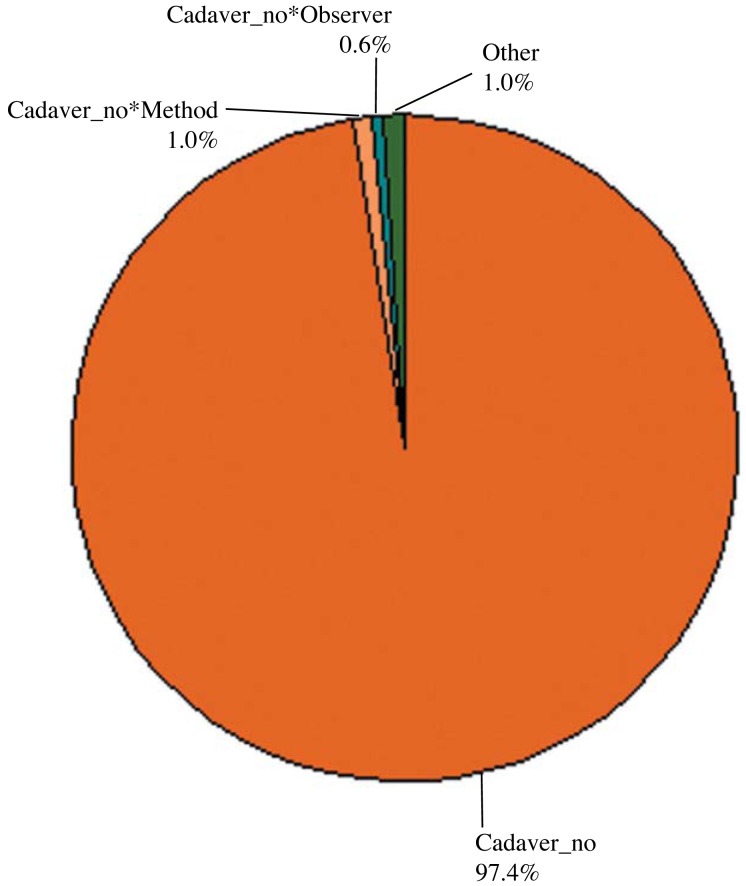
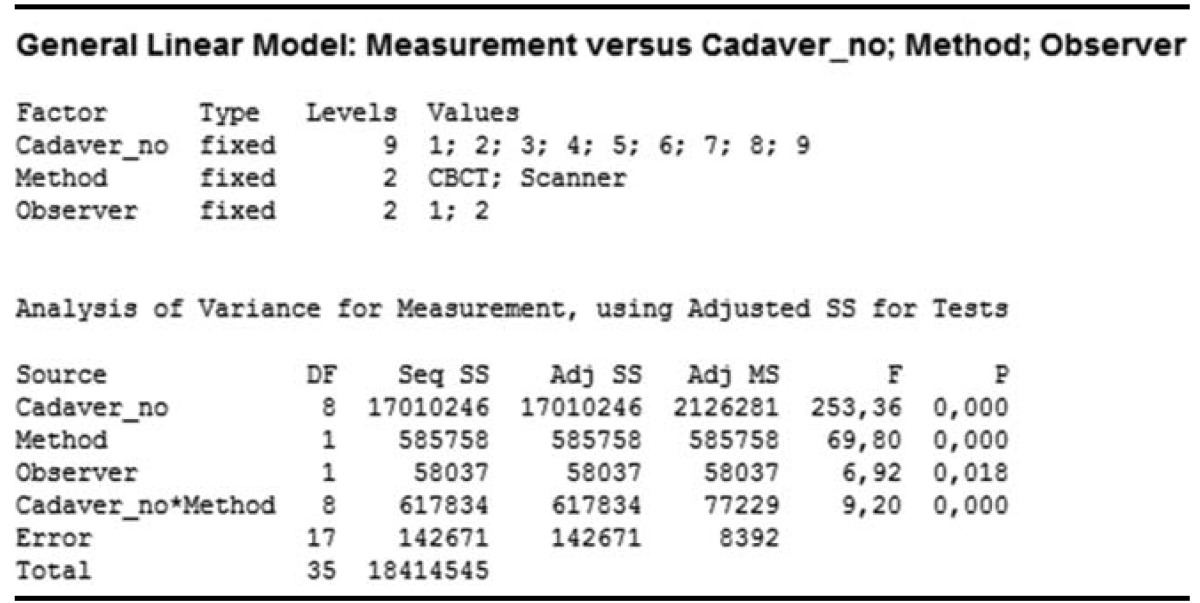
A comparison of FOVs according to observers revealed that the p values were smaller than 0.05 (p=0.009) for different FOVs and observers. However, this variation in the measurements was mainly attributed to the different cadaver specimens used. Comparisons of different FOVs on the basis of observers are presented in Table 1 and Figure 5. There was a significant difference between both observers, but among the total variation of FOV values, this difference contributed to a clinically small percentage. The variation among observers for different FOVs ranged between 0 and 10000mm3 with an average of 994 mm3, which could be considered clinically insignificant.
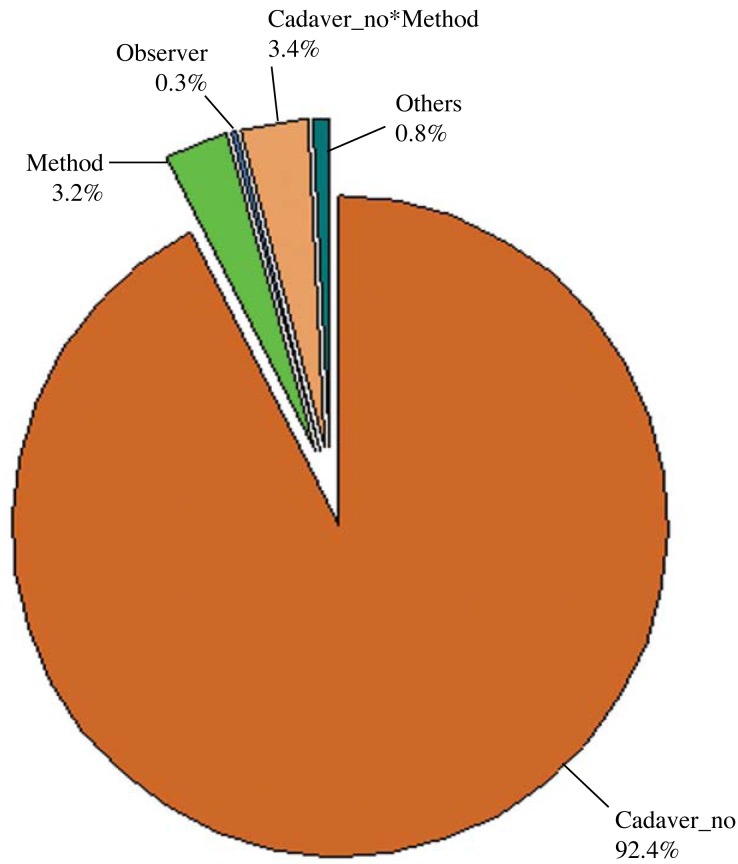
Only CBCT (FOV60) images were used for the comparison of CBCT and 3D optical scanner methods according to the observers and the gold standard. A comparison of the results obtained by the observers and the methods used in this study revealed that the p values were smaller than 0.05, suggesting that the measurement variations were caused by both methods and observers along with the different cadaver specimens used. The comparison of CBCT and 3D optical scanner methods on the basis of the observers and the gold standard is presented in Table 2 and Figure 6.
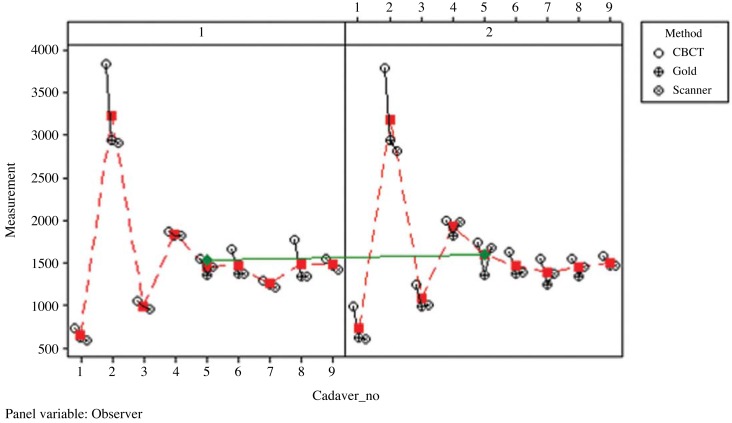
For both observers, 3D scanner measurements were closer to the gold standard measurements compared to the CBCT measurements. The multi-vari chart for measurement by method/observer is shown in Figure 7.
3D computed tomography (CT) models of jaw defects have the potential to play a significant role in prosthodontics and are a relatively new trend in dentistry. Optical scanners that form 3D computer models have been verified to enable sufficient system accuracy for clinical practice.7 Boldt et al.8 generated models of an edentulous maxilla by photo-optical, laser-optical, CT-based, and tactile means and compared the various findings to a reference plaster cast. Barone et al.9 stated that the accuracy and resolution of the optical scanner as compared to CBCT scanning allows optimal reconstructions of both tooth crown surfaces and oral soft tissues.
Several studies on the accuracy of intraoral scanners and digital impressions have been published, reporting single-unit restorations, several teeth in a row, quadrants, and full arch scans.1 Motohashi and Kuroda10 developed a 3D computer-aided system and scanned dental study models with a slit-ray laser beam, and Lu et al.11 introduced a laser scanning 3D digitization system for dental casts by using a special semiconductor laser. Hirogaki et al.12 scanned dental casts by using a line laser scanner and compared the measurements taken on computer-reconstructed models with those taken on actual casts. The difference was within 0.3 mm, which was in accordance with our results.
Several quantitative studies have been carried out on the use of CBCT systems and software in dental volumetric analysis. A study compared volumes of the extraction sockets by using software-guided segmentation estimated from CBCT images with the values obtained using Archimedes' principle; this previous study reported a good match between results similar to ours.13
The clinical use of 3D surface models obtained from CBCT includes pre-operative implant planning, assessment of the jaws, evaluation of the bone volume required for orthognathic surgery, and generation of physical dental models of the jaws by using stereolithography technology.14,15 The accuracy of a digitized model is restricted by the accuracy of the dental impression and cast, which could be variable and incoherent over time based on several factors. In practice, several patient scanning and data reconstruction parameters influence the subjective CBCT image quality. All these factors may also influence the quality of the 3D surface models reconstructed from CBCT images.16,17
The variation in the threshold value was less in the mandible than in the maxilla. This can be explained by the fact that the cortical bone in the mandible is sufficiently thick to keep the attenuation profile uniform across the entire bone surface, while in the maxilla, the varied thin cortical bone, particularly in the palate and tuberosity regions, creates the effect of bone dehiscence and fenestration artifacts in the 3D model.18
Other reports showing the use of CBCT for estimating the volume of teeth, pulp chamber, mandibular condyle, and upper airway volume also exist.19 In studies analyzing the effect of section thickness on volume estimations of organs or cavities by using CT images, the authors reported underestimations of the volumes, which were caused by increases in the slice thickness.20,21
The accuracy of segmentation relies on the gray value and the threshold value entered by the operator. Unfortunately, CBCT scanners do not have calibrated Hounsfield unit values. This implies that the gray values can differ for each scanner. In some previous studies, researchers used a threshold set automatically and independent of the operator.18,22 However, another study found the automated segmentation to have a greater error in assessing the volume of the lesions than manual readings (2% and 0.4%, respectively).23 Ahlowalia et al.24 compared the efficacy of micro-CT and CBCT on the measurement of bone defect volumes. Their results indicated that both CBCT and micro-CT showed a high degree of agreement when compared to the "Archimedes' principle" measurements.
Weissheimer et al.25 compared six different imaging software programs, namely Mimics, Dolphin3D, ITK-Snap, OsiriX, InVivo Dental, and Ondemand3D, for measuring the upper airway volume. All six imaging software programs were reliable but had errors in the volume segmentations of the oropharynx. Mimics, Dolphin3D, ITK-Snap, and OsiriX were similar (showed less than 2% errors) and more accurate than InVivo Dental and Ondemand3D (showed more than 5% errors) for the upper airway assessment.25 In the present study, we preferred the use of 3D Doctor and VRMesh Design since both of them were appropriate for our study design. According to company information, 3D Doctor uses a unique vector-based technology for better 3D mesh model creation and easy editing. In addition, the surface model uses a relatively small number of triangles while maintaining all details for high-quality rapid-prototyping applications. On the other hand, VRMesh Design offers the advantages of automatic point cloud classification, advanced point cloud decimation and denoising, accurate point cloud triangulation, and advanced stereolithography (STL) file repair and editing.
In conclusion, in the assessment of artificially created maxillary defects, we found clinically insignificant differences between observers and CBCT FOVs; however, 3D optical scanner measurements were more accurate than the CBCT measurements. Our findings might be useful for developing a reproducible, reliable, and useful clinical method to create accurate virtual models for the fabrication of obturating reconstructive prostheses.
Acknowledgments
We would like to express our sincere gratitude to Ayberk Yagiz of Ay Tasarim, Ankara, Turkey, for his assistance with this study.
References
1. Yuzbasıoglu E, Kurt H, Turunc R, Bilir H. Comparison of digital and conventional impression techniques: evaluation of patients' perception, treatment comfort, effectiveness and clinical outcomes. BMC Oral Health. 2014; 14:10. PMID: 24479892.

2. Lethaus B, Kessler P, Boeckman R, Poort LJ, Tolba R. Reconstruction of a maxillary defect with a fibula graft and titanium mesh using CAD/CAM techniques. Head Face Med. 2010; 6:16. PMID: 20642821.

3. Angelopoulos C, Scarfe WC, Farman AG. A comparison of maxillofacial CBCT and medical CT. Atlas Oral Maxillofac Surg Clin North Am. 2012; 20:1–17. PMID: 22365427.

4. Scarfe WC, Farman AG, Levin MD, Gane D. Essentials of maxillofacial cone beam computed tomography. Alpha Omegan. 2010; 103:62–67. PMID: 20645632.

5. Scarfe WC, Farman AG. What is cone-beam CT and how does it work? Dent Clin North Am. 2008; 52:707–730. PMID: 18805225.

6. Scarfe WC, Li Z, Aboelmaaty W, Scott SA, Farman AG. Maxillofacial cone beam computed tomography: essence, elements and steps to interpretation. Aust Dent J. 2012; 57(Suppl 1):46–60. PMID: 22376097.

7. Kamegawa M, Nakamura M, Fukui Y, Tsutsumi S, Hojo M. Direct 3-D morphological measurements of silicone rubber impression using micro-focus X-ray CT. Dent Mater J. 2010; 29:68–74. PMID: 20379015.

8. Boldt F, Weinzierl C, Hertrich K, Hirschfelder U. Comparison of the spatial landmark scatter of various 3D digitalization methods. J Orofac Orthop. 2009; 70:247–263. PMID: 19484417.

9. Barone S, Paoli A, Razionale AV. Creation of 3D multi-body orthodontic models by using independent imaging sensors. Sensors (Basel). 2013; 13:2033–2050. PMID: 23385416.

10. Motohashi N, Kuroda T. A 3D computer-aided design system applied to diagnosis and treatment planning in orthodontics and orthognathic surgery. Eur J Orthod. 1999; 21:263–274. PMID: 10407535.

11. Lu P, Li Z, Wang Y, Chen J, Zhao J. The research and development of noncontact 3-D laser dental model measuring and analyzing system. Chin J Dent Res. 2000; 3:7–14. PMID: 11314539.
12. Hirogaki Y, Sohmura T, Satoh H, Takahashi J, Takada K. Complete 3-D reconstruction of dental cast shape using perceptual grouping. IEEE Trans Med Imaging. 2001; 20:1093–1101. PMID: 11686444.

13. Agbaje JO, Jacobs R, Michiels K, Abu-Ta'a M, van Steenberghe D. Bone healing after dental extractions in irradiated patients: a pilot study on a novel technique for volume assessment of healing tooth sockets. Clin Oral Investig. 2009; 13:257–261.

14. Turbush SK, Turkyilmaz I. Accuracy of three different types of stereolithographic surgical guide in implant placement: an in vitro study. J Prosthet Dent. 2012; 108:181–188. PMID: 22944314.

15. Pohlenz P, Blessmann M, Blake F, Gbara A, Schmelzle R, Heiland M. Major mandibular surgical procedures as an indication for intraoperative imaging. J Oral Maxillofac Surg. 2008; 66:324–329. PMID: 18201617.

16. Katsumata A, Hırukawa A, Okumura S, Naitoh M, Fujishita M, Ariji E, et al. Effects of image artifacts on gray-value density in limited-volume cone-beam computerized tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 104:829–836. PMID: 17448704.

17. Kwong JC, Palomo JM, Landers MA, Figueroa A, Hans MG. Image quality produced by different cone-beam computed tomography settings. Am J Orthod Dentofacial Orthop. 2008; 133:317–327. PMID: 18249300.

18. Hassan B, Couto Souza P, Jacobs R, de Azambuja Berti S, van der Stelt P. Influence of scanning and reconstruction parameters on quality of three-dimensional surface models of the dental arches from cone beam computed tomography. Clin Oral Investig. 2010; 14:303–310.

19. Sezgin OS, Kayıpmaz S, Sahin B. The effect of slice thickness on the assessment of bone defect volumes by the Cavalieri principle using cone beam computed tomography. J Digit Imaging. 2013; 26:115–118. PMID: 22539100.

20. Emirzeoglu M, Sahin B, Selcuk MB, Kaplan S. The effects of section thickness on the estimation of liver volume by the Cavalieri principle using computed tomography images. Eur J Radiol. 2005; 56:391–397. PMID: 15893441.

21. Sahin B, Mazonakis M, Akan H, Kaplan S, Bek Y. Dependence of computed tomography volume measurements upon section thickness: an application to human dry skulls. Clin Anat. 2008; 21:479–485. PMID: 18627101.

22. Loubele M, Maes F, Schutyser F, Marchal G, Jacobs R, Suetens P. Assessment of bone segmentation quality of conebeam CT versus multislice spiral CT: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102:225–234. PMID: 16876067.

23. Pinsky HM, Dyda S, Pinsky RW, Misch KA, Sarment DP. Accuracy of three-dimensional measurements using conebeam CT. Dentomaxillofac Radiol. 2006; 35:410–416. PMID: 17082331.

24. Ahlowalia MS, Patel S, Anwar HM, Cama G, Austin RS, Wilson R, et al. Accuracy of CBCT for volumetric measurement of simulated periapical lesions. Int Endod J. 2013; 46:538–546. PMID: 23216253.

25. Weissheimer A, Menezes LM, Sameshima GT, Enciso R, Pham J, Grauer D. Imaging software accuracy for 3-dimensional analysis of the upper airway. Am J Orthod Dentofacial Orthop. 2012; 142:801–813. PMID: 23195366.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download