This article has been
cited by other articles in ScienceCentral.
Abstract
Skin prick tests (SPTs) are widely used to demonstrate an IgE-mediated hypersensitivity reaction to a specific allergen. However, local allergic conditions cannot be diagnosed with SPTs. Local specific IgE production was only presented before in mucosal tissues. We present a patient with house dust mite sensitization that had variable SPTs results in different body regions.
Keywords: Skin prick tests, Local allergy, Entopy, Disease mechanism
INTRODUCTION
Skin prick tests (SPTs) demonstrate an immediate, IgE-mediated immune response to a specific allergen and are the primary tools for allergy diagnosis. Guideline recommendations about the quality and potency of the extracts, application and interpretation of SPTs are well defined. SPTs yield useful evidence to demonstrate specific allergy to inhalant allergens when it is done with experienced personnel, appropriate equipment and high quality extracts [
1]. However, it has a low diagnostic accuracy for recently described local allergy syndromes that course with local mucosal allergen specific IgE production. Local allergic rhinitis (LAR=entopy) is described as local production of allergen specific IgE in nasal mucosal cells, that is not of blood or lymphatic origin. Although LAR is the most researched entity, prior studies have shown that local antigen specific IgE can be detected in esophagus, sinus mucosa and tears besides nasal mucosa [
2]. However, as far as we know, there is no data about demonstration of local allergen specific IgE in nonmucosal tissues. Herein, we want to present an incidentally identified interesting case with possible local inhalant allergen specific IgE presence in a nonmucosal tissue.
CASE REPORT
Thirty-nine-year-old female presented with a history of perennial rhinorrhoea, nasal obstruction, sneezing, and ocular symptoms for years. She had no other known comorbidities. She used intranasal corticosteroids (INCS) and oral H
1-antihistamines (OAH) irregularly. SPTs (No. 1) showed
Dermatophagoides farina (DF), and
Dermatophagoides pteronyssinus (DP) sensitivity (
Table 1). Complete blood count was normal. She was diagnosed with severe persistent allergic rhinitis. Allergen avoidance was advised, and INCS and OAH were prescribed. At third month of her therapy, since she was still symptomatic with high dose INCS and OAH, subcutaneous immunotherapy was planned. At that time, due to a misunderstanding, SPTs was performed as panel A (No. 2) to one arm and panel B (No. 3) to the other arm, simultaneously. The tests were performed by the same personnel, the same technique, the same type of lancet, and the same brand of extracts which were not expired. Results showed positive DF and DP with panel A on the right arm and negative DF and DP with panel B on the left arm. She had no skin lesions, comorbidities, any kind of topical medicine application history at the test sites, or recent anaphylaxis history that may explain this discordance. Therefore, extracts of panel A was applied to the left arm as well (No. 4) but the tests were negative again. In order to enlighten these results, serum mite specific IgEs and phadiatop were studied. One month later, after OAH discontinuation, informed consent was obtained from the patient and test numbers 5, 6, 7, and 8 seen in the
Table 1 were reperformed by the same personnel, the same bottle of extracts and the same technique. Panel A was negative on the left arm and only DF was positive on the right arm. DP and DF of panel B were negative and DP of panel A was positive on the dorsal skin (
Fig. 1). Mite specific IgEs and phadiatop were found negative (<0.35 kU/L).
DISCUSSION
False skin tests may result due to inexperienced personnel, wrong technique, nonstandardized, expired or low qualified extracts. In our case, all tests except for numbers 1, 3, and 8 were performed by the same lancet and the same technique with same brand, and non-expired, same bottles of extracts that were kept under optimum conditions. DFs of both panels had the same batch number in all tests except for number 1. The fact that there was no positivity observed with panel B might initially points to a problem with the quality of allergen. However, positive results with the same panel B bottle were reported in different patients, so we excluded this possibility. There was also positivity with positive control in every test, which indicates that there is no local anergy or similar underlying condition. Negative results in left arm and right dorsal area—which have no lymphogenic or dermatomal relationship—led us to rule out other possible conditions that may reduce SPT reactivity.
Unlike LAR, our findings are observed in skin, which normally blocks the passage of high molecular particles as dust mite, unless there is no defect in its barrier function. Also recent researches reported an association between skin barrier disruption and cutaneous sensitization; we think that this evidence cannot explain the diversity of SPT results in our case [
3].
As it's explained above, after excluding other possible reasons like comorbidities, improper technique or quality issue of the extracts, hypothetically we think that the nonhomogeneous expression of tissue mast cells or mite specific IgEs bound to tissue mast cells may explain the possible underlying mechanism. As far as we know, there is no data that may explain whether this is possible in humans. Unfortunately, we do not have the proper infrastructure to prove that hypothesis either by pathological and immunological or provocation tests. For now, it is not clear whether nasal allergen specific IgE production in LAR is secondary to local sensitization or a spontaneous immune response. It is shown that nasal allergen specific IgE may be found in about 50% of the healthy control subjects and we believe that our hypothesis may bring a new point of view to the conflicting concept of LAR [
4]. Although our case also states the importance of the objective demonstration of allergen sensitization in different skin areas which will enable anti-IgE treatment opportunity in patients with severe asthma and rhinitis with low levels of specific IgEs and negative SPTs. Additionally, in patients with typical allergic symptoms and negative serum specific IgEs and negative SPTs results, revealing a possible SPT positivity in a different skin area may also provide a chance for allergen immunotherapy. We believe advanced further molecular and histopathological studies are needed in order to explain if our hypothesis is possible or not.
Figures and Tables
Fig. 1
Skin prick tests (SPTs) results (numbers 7 and 8) of the dorsal skin of the patient. Dermatophagoides pteronyssinus (DP) and Dermatophagoides farina (DF) of panel B were negative and DP of panel A was positive. GH, panel A histamine; GN, panel A negative control; G3, panel A DF; G4, panel A DP; AH, panel B histamine; AN, panel B negative control; A3, panel B DF; A4, panel B DP.
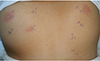
Table 1
Skin tests results of the patient







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download