CASE REPORT
A 56-year-old alcoholic man was transferred to the emergency room with a history of confusion, motor weakness, and gait disturbance for a few days. He had been drinking more than one bottle of soju every day for 30 years and stopped drinking 4 days ago. After he stopped drinking, the above symptoms occurred. His caloric food intake was poor, and he had consumed only alcohol in the preceding 15 days before quitting drinking. He already had alcoholic liver cirrhosis, Wernicke's encephalopathy, and alcoholic dementia.
The initial vital signs revealed a blood pressure (BP) of 110/68 mmHg, pulse of 88 bpm, respiratory rate of 20 breaths per minute, and temperature of 36.6°C. On initial neurological examination, the patient could answer only simple one-step obey commands, and he was only oriented to place. His pupils were isocoric and pupil light reflex was prompt. Other cranial nerve function tests did not show any abnormal findings. The Glasgow Coma Scale was 13/15 (eye scores, 4; verbal scores, 5; and motor scores, 4).
Initial laboratory data revealed severe hypernatremia (serum sodium level, 164 mEq/L), hypokalemia (potassium level, 2.9 mEq/L), anemia (hemoglobin level, 6.9 g/dL), and abnormal liver function (aspartate transaminase level, 145 IU/L).
Brain computed tomography (CT) was negative for acute hemorrhage or edema. He was admitted to the internal medicine department to focus on treatment for anemia, chronic obscure bleeding, electrolyte imbalance, and acute kidney injury (blood urea nitrogen [BUN]/creatinine [Cr], 52.9/1.20). After 3 days, his mental status changed from drowsy to stupor, and he developed respiratory failure and was attached to a ventilator.
Brain magnetic resonance imaging (MRI) showed high signal intensities symmetrically in the bilateral pons, basal ganglia, thalamus, and cerebral and cerebellar cortices on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images. Diffusion-weighted imaging (DWI) showed marked hyperintense lesions corresponding to the areas of low signal intensity on quantitative apparent diffusion coefficient (ADC) images (
Fig. 1). It was compatible with CPM and EPM. Finally, the patient died of complicated pneumonia with acute respiratory failure.
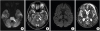 | Fig. 1
MRI of the brain demonstrating central pontine (arrow) hyperintensity in a diffusion-weighted image (A) and T2-weighted image (B). A diffusion-weighted image (C) and ADC image (D) show increased signal intensity in the bilateral basal ganglia, thalami, and cerebral cortices.
MRI, magnetic resonance imaging; ADC, apparent diffusion coefficient.

|
DISCUSSION
CPM and EPM are the radiological manifestations of osmotic myelinolysis, affecting different brain regions. The radiological manifestations are confined to the pons in cases of CPM and to the extrapontine sites in cases of EPM. The lesions are mostly symmetrical. Frequently affected parts in ODS are the pons in CPM and the cerebellum, lateral geniculate body, external capsule, hippocampus, putamen, cerebral cortex, thalamus, and caudate nucleus and more infrequently, the claustrum, internal capsule, midbrain, and internal medullary lamella, in EPM [
3]. EPM is more commonly observed with hypernatremia [
1]. In particular, hippocampal involvement may be related to the high vulnerability of the hippocampus to the neurotoxic effects of osmotic derangement and general systemic stress [
1].
Associations between hyponatremic osmotic disturbances and cerebral lesions are well-studied. In 1979, Messert et al. [
5] reported on the concept of the grid theory, i.e., the basis pontis consists of grid fibers which are vulnerable to cerebral edema because of declined elasticity with tegmentum, which lacks the grid fibers, preservation. The worse edema of the grid, the worse strangulation of myelin sheaths and blood vessels leading to demyelination, necrosis, and death. This theory is supported by the sparing of the dorsal pons or pontine tegmentum.
It has been increasingly recognized that EPM can occur in the setting of other osmotic challenges, such as glycemic state and end-stage renal disease regardless of serum sodium levels [
1]. According to a recent report, clinical symptoms do not correlate with age, the presence of comorbid conditions, initial serum Na
+ levels, serum osmolality, or the serum Na
+ correction rate [
1]. Apart from correction rates, the presence of additional metabolic derangements, such as serum K
+ levels and hyperglycemia, are considered to be independent risk factors for the development of ODS [
1]. Therefore, an increase in serum sodium, as well as potassium levels, should not exceed 12 mEq/L in the first 24 hours and 18 mEq/L in the first 48 hours [
4]. In addition, severe hypophosphatemia most likely also contributes to the development of EPM [
6].
In cases of hypernatremia, osmotic differences are subtle because of the rapid ionic and water shifts across the cell membrane with little effect on cell volume but a significant effect on decreased extracellular space volume [
1]. Blood-brain barrier (BBB) disruption occurs secondary to osmotic stress and is one of the leading factors in the pathogenesis of EPM [
3]. Compared with patients with no comorbid conditions, the presence of indirect central nervous system (CNS) comorbid conditions, such as alcoholism, especially alcohol withdrawal, malnutrition, diuretic use, burns, post-liver transplantation, and post-partum dehydration status, before hypernatremic challenge renders the brain more vulnerable to ODS with mildly elevated sodium levels [
3].
MRI is the imaging technique of choice for the diagnosis of ODS because it has a greater sensitivity for CPM than CT and a superior capacity for demonstrating EPM lesions. CPM is characterized by hyperintensity on T2-weighted, FLAIR, DWI imaging, and hypointensity on T1-weighted, ADC imaging [
1]. But EPM shows variable images, not characteristic or typical ones. In this case, brain MRI images corresponded with typical radiological findings of CPM and extrapontine lesions were showed the same results. One study reported that early DWI changes are a common finding in ODS but do not regularly precede the tissue changes that are detectable on conventional MRI sequences [
2]. With these radiologic findings, neurologic examinations, laboratory findings, and past history, we finally diagnosed ODS and could differentiate from other possible diseases.
The prognosis of ODS varies. Early reports on CPM indicated approximately 100% mortality rate within 3 months following hospital admission [
7]. The most recent large series of 34 cases showed that only 2 patients died, 10 survived but were left dependent, 11 had some deficits but were independent, and 11 recovered completely [
8]. Pediatric patients have a better recovery rate, and the associated MRI lesions are more reversible [
9]. Younger patients had a worse outcome than older patients.
Common electrolyte imbalance associated with chronic alcoholics is hypomagnesia, hyponatremia, hypocalcemia, and hypophosphatemia. Hyponatremia has been reported to be more common than hypernatremia. The mechanism of hyponatremia is below; the lowering of secretion of antidiuretic hormone (ADH) by alcohol cause excessive urination. However, the minimum of 50 to 60 mOsmol of solutes is necessary to dilute a maximum of 1 L urine. Drinking low alcohol concentration liquor, such as beer, may cause hyponatremia (beer potomania syndrome) because the relatively huge amount of free water is over-absorbed than that of excreted solutes. However, drinking high alcohol concentration liquor may cause volume depletion, resulting in hypernatremia. So, reset osmostat syndrome can be developed in chronic alcoholics, which is inappropriate natriuresis (> 40 mmol/L) despite serum hyponatremia.
CPM can be commonly developed in chronic alcoholics. Malnutrition and hypokalemia have been suggested for predisposing factors [
10].
Previous reports have highlighted the importance of serum Na+ correction in ODS associated with hyponatremia in chronic alcoholics, however, there have been very few case reports of ODS with hypernatremia. Especially during alcohol withdrawal, there was not reported case associated with hypernatremia. In our case, CPM and EPM were observed simultaneously after correction of hypernatremia, and it was assumed that chronic alcoholism and quitting drinking caused brain structural changes which are vulnerable to get an injury to change of serum sodium level.
Therefore, this case suggests that ODS easily occurs, particularly in at-risk patients, such as known alcoholics who are still drinking or stops drinking within a few days, malnourished patients, and chronic patients with liver and kidney diseases, whether or not abnormal serum sodium level is present. ODS should be included in the differential diagnosis of patients who manifest with new psychotic symptoms during alcohol withdrawal. It is not difficult to distinguish ODS from Wernicke's encephalopathy through determination of whether nystagmus is present, improvement with thiamine supplementation, or signal changes on MRI. Management of these conditions involves recognition of at-risk patients, careful electrolyte correction, prompt and exact diagnosis, and management of associated complications with early rehabilitation for functional recovery.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download