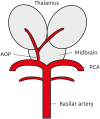
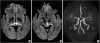
1. Percheron G. The anatomy of the arterial supply of the human thalamus and its use for the interpretation of the thalamic vascular pathology. Z Neurol. 1973; 205:1–13.
2. Carrera E, Michel P, Bogousslavsky J. Anteromedian, central, and posterolateral infarcts of the thalamus: three variant types. Stroke. 2004; 35:2826–2831.
3. Kumral E, Evyapan D, Balkir K, Kutluhan S. Bilateral thalamic infarction. Clinical, etiological and MRI correlates. Acta Neurol Scand. 2001; 103:35–42.
4. Reilly M, Connolly S, Stack J, Martin EA, Hutchinson M. Bilateral paramedian thalamic infarction: a distinct but poorly recognized stroke syndrome. Q J Med. 1992; 82:63–70.
5. Blumenfeld H. Neuroanatomy through clinical cases. 2nd ed. Sunderland (MA): Sinauer Associates;2010.
6. Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003; 34:2264–2278.
7. Castaigne P, Lhermitte F, Buge A, Escourolle R, Hauw JJ, Lyon-Caen O. Paramedian thalamic and midbrain infarct: clinical and neuropathological study. Ann Neurol. 1981; 10:127–148.
8. Gentilini M, De Renzi E, Crisi G. Bilateral paramedian thalamic artery infarcts: report of eight cases. J Neurol Neurosurg Psychiatry. 1987; 50:900–909.
9. Bogousslavsky J, Regli F, Uske A. Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology. 1988; 38:837–848.
10. Arauz A, Patiño-Rodríguez HM, Vargas-González JC, Arguelles-Morales N, Silos H, Ruiz-Franco A, et al. Clinical spectrum of artery of percheron infarct: clinical-radiological correlations. J Stroke Cerebrovasc Dis. 2014; 23:1083–1088.
11. Adamczyk P, Mack WJ. The artery of percheron and etiologies of bilateral thalamic stroke. World Neurosurg. 2014; 81:80–82.
12. Jodar M, Martos P, Fernández S, Canovas D, Rovira A. Neuropsychological profile of bilateral paramedian infarctions: three cases. Neurocase. 2011; 17:345–352.
13. Almamun M, Suman A, Arshad S, Kumar SJ. A case of midbrain and thalamic infarction involving artery of percheron. J Clin Med. 2015; 4:369–374.
14. Clark JM, Albers GW. Vertical gaze palsies from medial thalamic infarctions without midbrain involvement. Stroke. 1995; 26:1467–1470.
15. Barnes MP, Dobkin BH, Bogousslavsky J. Recovery after stroke. Cambridge: Cambridge University Press;2005.
16. Kim SJ, Yeom MI, Lee SU. A case of double depressor palsy followed by pursuit deficit due to sequential infarction in bilateral thalamus and right medial superior temporal area. Int Ophthalmol. Forthcoming. 2016.