Abstract
Purpose
Calprotectin is a cytosolic protein with immunomodulatory, antimicrobial, and antiproliferative actions. The concentration of calprotectin increases in infection, inflammation, and malignancy. We determined if calprotectin can be used as a marker for the diagnosis and follow-up of bowel inflammation in cow's milk protein allergy (CMPA).
Methods
In total, 32 patients newly diagnosed with CMPA were included (24 IgE-mediated, 8 non-IgE-mediated). In all subjects, a complete blood count, total IgE, cow's milk-specific IgE, and fecal calprotectin (FC) were assessed before and after a cow's milk protein (CMP) elimination diet was started. The results were compared with those of 39 healthy children.
Results
The mean FC value before the CMP elimination diet was 516±311 µg/g in the 32 patients with CMPA and 296±94 µg/g in the control group (P=0.011). The mean FC value after the diet in these patients was 254±169 µg/g, which was significantly different from the mean value before the CMP elimination diet (P<0.001). When we compared FC levels before the CMP elimination diet in the IgE-mediated group with the control group, we found no significant statistical difference (P=0.142). The mean FC value before the CMP elimination diet was 886±278 µg/g in the non-IgE-mediated group and 296±94 µg/g in the control group; this difference was statistically significant (P<0.001). In the IgE-mediated and non-IgE-mediated groups, FC values after CMP elimination diet were 218±90 µg/g and 359±288 µg/g, respectively, and FC values before CMP elimination diet were 392±209 µg/g and 886±278 µg/g, respectively; these differences were statistically significant (P=0.001 and P=0.025, respectively).
Children are the major age group affected by cow's milk protein allergy (CMPA).1 The symptoms that suggest CMPA are observed in 5%-15% of infants; however, the incidence of CMPA is approximately 2%-5% when specific diagnostic criteria are used. In most patients, the symptoms develop before 1 year of age and within 1 week after the intake of cow's milk.2,3 Food allergies are classified in 2 groups: IgE-mediated and non-IgE-mediated allergy.4 Some reactions may include both mechanisms (mixed type).5
Skin prick tests and serum specific IgE tests can help determine a diagnosis in all patients. However, both tests are not required at the same time in all patients and either can be sufficient in a given patient.6,7 In particular, an oral challenge test is usually necessary for the diagnosis of non-IgE-mediated allergy.8 Usually, a specific IgE test is negative in patients with gastrointestinal symptoms; however, the diagnosis of CMPA should not be excluded even if a specific IgE test is negative in patients who have skin lesions.8,9
Calprotectin is a cytosolic protein that belongs to the S-100 protein group that increases under conditions such as inflammation, infection, and malignancy. Fecal calprotectin (FC) may increase under many conditions such as inflammatory bowel diseases (IBDs). Calprotectin can also be measured in plasma, synovial fluid, cerebrospinal fluid, oral fluids and urine. Calprotectin is a calcium and zinc binding protein. It decreases the local zinc intensity by binding to zinc. In this way, it deprives microorganisms of zinc and additionally inhibits many zinc-dependent enzymes.10-14 Calprotectin is a cytosolic protein with immunmodulatory, antimicrobial and antiproliferative action that is predominatly found in neutrophils, monocytes and macrophages as well as (to a lesser extent) in T and B lymphocytes. The measurement of FC levels is a sensitive and non-invasive marker that determines an active inflammation in the gastrointestinal system of pediatric patients.15-17
FC release seems related to the passage of neutrophils and mononuclear cells to the intestinal wall, their turnover, and migration into the intestinal lumen. This theory is supported by the association of the excretion of indium-111-labeled neutrophils and FC intensity.18 FC intensity increases in children and adults with IBD. Increased FC levels in patients with IBD have been associated with increased migration of neutrophils towards the intestinal lumen in inflamed mucosa. FC levels in patients with IBD seem related to the activation and histological state of the disease; subsequently, calprotectin may be a useful marker to observe treatment responses and determin relapses.19,20
In this study, we determined if FC could be a marker for the diagnosis and disease follow-up in bowel inflammation associated with CMPA.
This study included 32 patients who were newly diagnosed with CMPA and followed at the Cerrahpaşa Medical Faculty Pediatric Gastroenterology and Pediatric Allergy outpatient clinic.
The diagnosis was in accordance to ESPGHAN Guideline: Diagnosis and Management of CMPA.7
All patients were diagnosed according to the protocol (Fig. 1).7 The first step was a thorough medical history review and physical examination. CMPA was suspected if any of the features listed below occurred in an infant or child and could not be explained by another cause: digestive (dysphagia, food impaction, colic, abdominal pain, frequent regurgitation, vomiting, anorexia, refusal to feed, diarrhea [intestinal protein loss, blood loss, or constipation], perianal rash, occult blood loss, iron-deficiency anemia, and failure to thrive), respiratory (runny nose, wheezing or stridor, and chronic coughing [all unrelated to infections]), dermatologic (urticaria, atopic eczema, and swelling of lips or eyelids), andsystemic (anaphylaxis, shock-like symptoms with severe metatobolic acidosis). We have first eliminated cow's milk proteins (CMP) from the diet of the patient group suffering from anaphylaxis or an immediate reaction (vomiting, wheezing or stridor, breathing difficulties, urticaria, angioedema, shock-like symptoms with severe metatobolic acidosis, vomiting and diarrhea; within minutes-2 h) after taking cow's milk or formula. After amelioration of these findings following the elimination, we have diagnosed the patients with CMPA. CMPA sensitivity was investigated with cow's milk-specific IgE. A therapeutic CMP elimination diet was given if the cow's milk-specific IgE was positive.
A standardized oral challenge with CMP was performed if the cow's milk-specific IgE was negative. A therapeutic CMP elimination diet was provided if the standardized oral challenge with CMP was positive. Patients did not have the diagnosis of CMPA if the standardized oral challenge with CMP was negative.
Diagnostic CMP elimination diet were performed on patients whose history and physical examination findings were suggestive of the diagnosis of CMPAwithout an anaphylaxis or immediate type reaction.
A standardized oral challenge with CMP was performed in patients who improved in the clinical symptoms by the elimination diet. A therapeutic CMP elimination diet was given if the standardized oral challenge with CMP was positive. Patients did not have a diagnosis of CMPA and were excluded from the study if the standardized oral challenge with the CMP was negative; in addition, patients did not have the diagnosis of CMPA if the patients showed no improvement during the clinical symptoms elimination diet.
The determination of specific IgE in a blood sample and the skin prick test are useful diagnostic tests at any age for clinical practice; however, a combination of the 2 tests is not necessary for the diagnostic workup.6,7 For this reason, CMPA sensitivity was investigated with only cow's milk-specific IgE for all patients.
Cow's milk-free diet was given to the patients with CMPA and their mothers if they were fed with breast milk.
In all subjects, a complete blood count, total IgE, cow's milk-specific IgE, and FC were tested before and after the diet was started.
The results were compared with the results for a pediatric group that consisted of 39 healthy children aged between 1 and 34 (11.5±7.6) months who had no gastrointestinal complaint and whose cow's milk-specific IgE was found to be negative. Healthy children with no allergic disease, food allergy, or gastrointestinal complaints were selected from the Cerrahpaşa Medical Faculty Well Child Outpatient Clinic and from the children of healthcare workers as the control group. Before fecal samples were taken, it was determined that no infectious disease, abdominal pain, or diarrhea was present and that the subjects were not receiving non-steroidal anti-inflammatory treatment. A complete blood count, cow's milk-specific IgE, total IgE, and FC were assessed.
FC was tested on the same day when the feces samples were obtained. A feces sample was weighed (50 mg) on an assay balance, 2.5 mL buffer solution was added, and the sample was vortex-mixed for 30 minutes. The samples were centrifuged (1,000 rpm, 5 minutes); subsequently, 1 mL supernatant was placed in a microcentrifuge tube and kept frozen at -20℃. The feces samples were tested using a Bühlman ELISA kit and a Biotech Instruments, Inc. ELX 800 (USA) ELISA device.
Cow's milk-specific IgE was tested using the UNICAP device (Phadia Austria GmbH Donau-City-Str. 1, AT-1220 VIENNA, Austria) and a fluorescent enzyme immunoassay (FEIA) method. A cow's milk-specific IgE value higher than 0.35 kU/L was considered positive. Total IgE was tested nephelometrically using a BN2 nephelometer device (Siemens, Munich, Germany). Serum total IgE was based on age-dependent values.
The study protocol was approved by the Ethics Committee of our institution. Written informed consent was obtained from the parents of all subjects.
Data were analyzed using SPSS 15 software. The Mann-Whitney U-test was used for independent statistical variables and the Wilcoxon test was used for dependent statistical variables. Variables with a normal distribution are expressed as means±standard deviations. Variables with a non-normal distribution are expressed as medians and ranges. A P-value of <0.05 was considered to indicate a statistical significance.
IgE-mediated CMPA was found in 24 patients and a non-IgE-mediated CMPA was found in 8 patients. Table 1 provides the demographic and general properties of the patients.
FC levels were significantly higher in patients with CMPA compared with the control group (P=0.011; Fig. 2). FC levels were significantly lower after than before the CMP elimination diet in these patients (P<0.001; Fig. 3).
Table 2 shows the comparison between the laboratory findings before the CMP elimination diet in patients with IgE-mediated CMPA and the laboratory findings in the control group. The FC value in the IgE-mediated CMPA group was higher than the control group; however, the difference was not statistically significant (P=0.142).
Table 3 shows the comparison of the laboratory values before and after the CMP elimination diet in patients with IgE-mediated CMPA. FC values were found to be significantly lower after the CMP elimination diet than before (P=0.001).
Table 4 shows the comparison of laboratory values before the CMP elimination diet in patients with non-IgE-mediated CMPA and those in the control group. FC values were significantly higher in the IgE-mediated group than in the control group (P<0.001).
Table 5 shows the comparison of the laboratory values before and after the CMP elimination diet in patients with non-IgE-mediated CMPA. FC values were significantly lower after the CMP elimination diet than before (P=0.025).
A study by Ezri et al.22 found that FC levels varied with age; the cut-off level in the first year of life (<350 µg/g) was higher than that during childhood (<275 µg/g) and adulthood (<50 µg/g). In our study, the mean FC value was 296±94 µg/g in 19 healthy children aged between 1 and 31 months (median: 311; minimum: 156; maximum: 475).
FC levels seem to be related to infant age. The mean FC value was 265 µg/g in infants aged between 0 and 3 months. This was thought to be related to the development of oral tolerance and the establishment of the microbial flora.23
Baldassarre et alcompared the FC levels of 30 CMPA patients with rectal bleeding and those of healthy infants of the same age.24 FC levels were markedly higher in CMPA patients than in the control group (325.89±152.31 vs. 131.97±37.98 µg/g, P<0.001). The FC level decreased by 50% after the elimination of cow's milk from the diet for 4 weeks; however, it was still higher than the control group (157.5±149.13 vs. 93.72±36.65 µg/g, P=0.03). This study showed that FC increased in CMPA patients with rectal bleeding and decreased with the cow's milk-elimination diet.
In a pilot study performed in six patients with cow's milk allergy, FC levels were measured at baseline and 3 and 6 weeks after protein hydrolysate formula was started. FC levels were 135-1,537 mg/L (mean: 557 mg/L) at baseline and decreased to 42-219 mg/L after 6 weeks (mean: 163 mg/L). Thus, FC could be useful to demonstrate a response to protein hydrolysate formula treatment.
In the present study, a comparison of FC levels before and after the CMP elimination diet in patients with CMPA revealed a significant decrease (P<0.001); in addition, the FC level before the CMP elimination diet was significantly higher than in the control group (P=0.011).
FC levels before the CMP elimination diet were higher in the IgE-mediated CMPA group (n=24) compared with the control group and the difference was not statistically significant (P=0.142). However, a statistically significant difference was found between FC levels before and those after the CMP elimination diet (P=0.001). Thus, FC levels may be useful in treatment follow-up for the IgE-mediated group.
FC levels were found to be higher before the CMP elimination diet in the non-IgE-mediated CMPA group (n=9) than in the control group; the difference was statistically significant (P<0.001). A statistically significant difference was found (P=0.025) when FC levels before and after treatment were compared; subsequently, FC may be useful for the follow-up of treatment and recurrence determination in the non-IgE-mediated group.
A comparison of the IgE-mediated and non-IgE-mediated groups revealed significantly higher FC levels in the non-IgE-mediated group. We considered that the FC level was higher compared with the IgE-mediated group because gastrointestinal symptoms and colitis are predominant in non-IgE-mediated CMPA patients. FC may be more useful to determine relapses and the follow up of patients in the non-IgE-mediated group becuase gastrointestinal involvement is more common in the CMPA patient population.
In various studies performed on children, different cut-off values have been used for FC. The FC value was 296±94 µg/g (10.5±7.6 months) in our study. We found relatively high values compared with those in the literature. FC was observed to decrease in response to treatment and with clinical remission compared with values at baseline. This suggested that tracking FC levels might reveal increases or reductions in disease activity if the comparisons were based on the baseline value for each individual; however, no reference range according to age is apparent. Thus, FC levels may be useful as an inexpensive, simple, and non-invasive test to demonstrate and assess disease activity in CMPA patients.
FC can increase in case of several inflammatory bowel conditions; therefore, we assert that FC can be useful only to determine relapses and follow ups after diagnosing patients as CMPA particularly with gastrointestinal involvement.
More studies are needed to determine the reference values for FC levels in children. FC levels may be a useful marker for follow-up treatment and recurrence determination in CMPA.
Figures and Tables
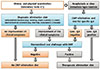 | Fig. 1Algorithm for infants and children with symptoms suggestive of cow's-milk protein allergy (CMPA). |
 | Fig. 2Comparison of fecal calprotectin levels before diet in CMPA patients with those in the control group. |
Table 1
Characteristics of patients with a diagnosis of CMPA (n=32)

Table 2
Comparison of laboratory findings before CMP elimination diet in patients with IgE-mediated CMPA with those in the control group
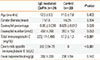
Table 3
Comparison of the laboratory findings before and after CMP elimination diet in patients with IgE-mediated CMPA
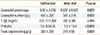
Table 4
Comparison of the laboratory values before CMP elimination diet in non-IgE-mediated CMPA patients with those in the control group
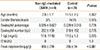
Table 5
Comparison of laboratory values before and after the CMP elimination diet in patients with non-IgE-mediated CMPA

References
1. Arvola T, Ruuska T, Keränen J, Hyöty H, Salminen S, Isolauri E. Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics. 2006; 117:e760–e768.
2. Hwang JB, Park MH, Kang YN, Kim SP, Suh SI, Kam S. Advanced criteria for clinicopathological diagnosis of food protein-induced proctocolitis. J Korean Med Sci. 2007; 22:213–217.
3. Eigenmann PA. Mechanisms of food allergy. Pediatr Allergy Immunol. 2009; 20:5–11.
4. Chehade M. IgE and non-IgE-mediated food allergy: treatment in 2007. Curr Opin Allergy Clin Immunol. 2007; 7:264–268.
5. Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004; 113:805–819.
6. NIAID-Sponsored Expert Panel. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010; 126:S1–S58.
7. Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, Mearin ML, Papadopoulou A, Ruemmele FM, Staiano A, Schäppi MG, Vandenplas Y. European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012; 55:221–229.
8. Klemola T, Vanto T, Juntunen-Backman K, Kalimo K, Korpela R, Varjonen E. Allergy to soy formula and to extensively hydrolyzed whey formula in infants with cow's milk allergy: a prospective, randomized study with a follow-up to the age of 2 years. J Pediatr. 2002; 140:219–224.
9. Eggesbø M, Botten G, Halvorsen R, Magnus P. The prevalence of CMA/CMPI in young children: the validity of parentally perceived reactions in a population-based study. Allergy. 2001; 56:393–402.
10. Berntzen HB, Olmez U, Fagerhol MK, Munthe E. The leukocyte protein L1 in plasma and synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. 1991; 20:74–82.
11. Dunlop O, Bruun JN, Myrvang B, Fagerhol MK. Calprotectin in cerebrospinal fluid of the HIV infected: a diagnostic marker of opportunistic central nervous system infection? Scand J Infect Dis. 1991; 23:687–689.
12. Cuida M, Brun JG, Tynning T, Jonsson R. Calprotectin levels in oral fluids: the importance of collection site. Eur J Oral Sci. 1995; 103:8–10.
13. Holt J, Fagerhol MK, Dale I. Quantitation of a leukocyte protein (L1) in urine. Acta Paediatr Scand. 1983; 72:615–616.
14. Isaksen B, Fagerhol MK. Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol Pathol. 2001; 54:289–292.
15. Canani RB, de Horatio LT, Terrin G, Romano MT, Miele E, Staiano A, Rapacciuolo L, Polito G, Bisesti V, Manguso F, Vallone G, Sodano A, Troncone R. Combined use of noninvasive tests is useful in the initial diagnostic approach to a child with suspected inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2006; 42:9–15.
16. Berni Canani R, Rapacciuolo L, Romano MT, Tanturri de Horatio L, Terrin G, Manguso F, Cirillo P, Paparo F, Troncone R. Diagnostic value of faecal calprotectin in paediatric gastroenterology clinical practice. Dig Liver Dis. 2004; 36:467–470.
17. von Roon AC, Karamountzos L, Purkayastha S, Reese GE, Darzi AW, Teare JP, Paraskeva P, Tekkis PP. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007; 102:803–813.
18. Røseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999; 34:50–54.
19. Fagerberg UL, Lööf L, Merzoug RD, Hansson LO, Finkel Y. Fecal calprotectin levels in healthy children studied with an improved assay. J Pediatr Gastroenterol Nutr. 2003; 37:468–472.
20. Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006; 12:524–534.
21. Nielsen RG, Fenger C, Bindslev-Jensen C, Husby S. Eosinophilia in the upper gastrointestinal tract is not a characteristic feature in cow's milk sensitive gastro-oesophageal reflux disease. Measurement by two methodologies. J Clin Pathol. 2006; 59:89–94.
22. Ezri J, Nydegger A. Pediatrics. Fecal calprotectin in children: use and interpretation. Rev Med Suisse. 2011; 7:69–70.
23. Olafsdottir E, Aksnes L, Fluge G, Berstad A. Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr. 2002; 91:45–50.
24. Baldassarre ME, Laforgia N, Fanelli M, Laneve A, Grosso R, Lifschitz C. Lactobacillus GG improves recovery in infants with blood in the stools and presumptive allergic colitis compared with extensively hydrolyzed formula alone. J Pediatr. 2010; 156:397–401.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download