Abstract
Background
Paraneoplastic limbic encephalitis (PLE) is a rare syndrome characterized by memory impairment, symptoms of hypothalamic dysfunction, and seizures. It commonly precedes the diagnosis of cancer. Small-cell lung cancer is the neoplasm that is most frequently reported as the etiology underlying PLE.
Case Report
This report describes a male patient who presented with neurologic symptoms consistent with anterograde amnesia, apathy, and disorientation. MRI revealed diffuse hyperintensities located predominantly in the medial bitemporal lobes, basal ganglia, frontal lobes, and leptomeninges on fluid attenuated inversion recovery images, suggesting PLE. Study of the primary tumor revealed squamous cell carcinoma of the lung. The patient was treated with neoadjuvant chemotherapy followed by surgery and adjuvant chemoradiotherapy, which resulted in his neurologic symptoms gradually improving.
Paraneoplastic limbic encephalitis (PLE) is one of the most frequent presentations of paraneoplastic encephalomyelitis syndrome. It is produced by involvement of the medial region of the temporal lobes, especially the hippocampus, which derive their characteristic clinical manifestations. By definition, PLE is associated with cancer, but it is not caused by a primary or metastatic neoplasia in the central nervous system (CNS). Therefore, the specific diagnosis of a PLE requires a cancer diagnosis.1
The differential diagnosis should be established, mainly to rule out infectious and autoimmune disorders (especially viral encephalitis and Whipple's disease). However, metabolic encephalopathies, neurotoxic drugs, deficiency states, inflammatory disorders, primary or secondary CNS tumors, and neurodegenerative disorders must be excluded.2,3,4 Hyperintensity in the temporal lobes, and especially in the medial region, is often evident on T2-weighted and fluid attenuated inversion recovery (FLAIR) sequences in MRI, and occasionally there is gadolinium enhancement.5 Among the many different neoplasms known to cause PLE, small-cell lung cancer (SCLC) is the most frequently reported etiology. We report herein a case of a PLE as a debut of squamous cell carcinoma of the lung.
A 54-year-old Caucasian male who smoked 20 cigarettes per day and had an otherwise unremarkable medical history was admitted to our hospital due to subacute neurologic symptoms. He presented with nocturnal myoclonus, sexual dysfunction, mood swings, insomnia, daytime sleepiness, and occasional disorientation. His vital signs were normal at the time of admission, and a physical examination did not detect any abnormality. A neurologic examination revealed anterograde amnesia, apathy, and a disorder index in the Mini-Mental State Examination of 24/30.
All blood test findings were within normal limits, and serology was negative for hepatitis B virus, hepatitis C virus, human immunodeficiency virus, leishmaniasis, histoplasmosis, syphilis, and borreliosis. The findings of immunological studies were normal for antinuclear antibodies, anti-DNA antibody, angiotensin-converting enzyme, complement, IgA, antineutrophil cytoplasmic autoantibodies, anti-Ro/La antibody, and anticardiolipin antibody; a proteinogram also yielded normal findings. A polymerase chain reaction performed using the cerebrospinal fluid (CSF) yielded negative findings for herpes simplex and varicella zoster virus. A cytologic analysis of the CSF did not reveal any malignant cells.
The CSF was also negative for paraneoplastic antibodies with specificity for recombinant Hu, Yo, Ri, Ta, amphiphysin, and Ma2 antigens. The electroencephalogram (EEG) was normal. Cerebral CT demonstrated hypodense lesions in the medial bitemporal region and in the right caudate nucleus, with pseudonodular uptake in both unci. MRI demonstrated diffuse hyperintensities in the parenchyma, with predominantly medial bitemporal involvement extending into the basal ganglia, frontal lobes, and leptomeninges on FLAIR images, which is the most characteristic sequence, suggesting a PLE (Fig. 1).
An extension study was conducted to locate a tumor. Testicular and prostate ultrasonography revealed slight changes suggestive of chronic prostatitis and normal gonads. CT of the abdomen and chest revealed a 33-mm nodule in the right inferior lobe (RIL), suggestive of hamartoma, and lymph nodes of significant size at stations 7 and 2R. Ultrasound endoscopy confirmed the presence of paraesophageal right hilar adenopathy, which was suggestive of malignancy. Histopathology of the lymph node was positive for squamous cell carcinoma. Fibrobronchoscopy demonstrated erythematous mucosa in the right superior lobe. Bronchoalveolar aspiration and bronchoalveolar lavage produced negative findings. Positron-emission tomography and CT (PET/CT) demonstrated a hypermetabolic lesion in the azygos-esophageal recess that have could represented a lymph-node conglomerate. Outbreaks of high metabolic activity in both hippocampal regions that were suggestive of limbic encephalitis were observed, but with no evidence of metastatic lesions.
The patient was diagnosed with squamous cell carcinoma of the lung TxN2M0 (stage IIIA). He was treated with three cycles of 75 mg/m2 cisplatin and 75 mg/m2 docetaxel every 3 weeks. A postreatment evaluation by PET/CT demonstrated a slight reduction of the mediastinal lesions. A right inferior lobectomy and a mediastinal lymphadenectomy were performed 3 months after completing the last cycle of chemotherapy. A pathologic examination demonstrated that the peribronchial, interlobar, and subcarinal lymph nodes contained squamous cell carcinoma with extension into the soft tissues and capsular rupture. The nodule in the RIL was confirmed as a pulmonary chondroid hamartoma. The surgical margins were free of disease. Brain FLAIR imaging after the surgery revealed remarkable decreases in both the extension and signal intensity within the medial region of both temporal lobes. Secondary mesial atrophy with temporal horns dilatation was observed (Fig. 2). The patient's neurologic symptoms improved after surgery. The postoperative therapy included 4 cicles of carboplatin (area under the curve 5) and 500 mg/m2 pemetrexed administered every 3 weeks with concomitant mediastinal radiotherapy.
After postoperative treatment, PET/CT revealed two hypermetabolic lesions in the right pleuroesophageal recess, and MRI revealed persistence of the T2-signal intensity and mesial bitemporal lobe atrophy. After 8 months of follow-up the patient presented with acute neurologic symptoms that required admission to an intensive care unit (ICU) due to fever, upper-extremity myoclonus, and somnolence. Brain MRI and CT demonstrated a persistent T2-elongation of the mesial regions and no definitive gadolinium enhancement. The CSF was positive for Escherichia coli and Staphylococcus aureus. An EEG revealed diffuse neuronal dysfunction with no focal or diffuse paroxysms suggestive of seizures. Despite treatment with broad-spectrum antibiotics, the patient had a low level of consciousness with persistent neurologic deterioration, and died 1 month after ICU admission. The autopsy revealed persistent squamous cell carcinoma in the tracheal and mediastinal lymph nodes with extensive infiltration of the esophagus wall and a tracheoesophageal fistula. Infiltration of the right atrium, right ventricle, and renal capsule was also observed. In the brain, the autopsy confirmed advanced bilateral T-cell-mediated limbic encephalitis with persistent inflammatory activity (Fig. 3) extending to the midbrain tectum.
Paraneoplastic limbic encephalitis is a rare disease characterized by the subacute onset of short-term memory loss, seizures, and symptoms of hypothalamic dysfunction. It commonly precedes a diagnosis of malignancy, and it is a diagnosis of exclusion. The association between subacute encephalopathy and a distant tumor was first described in 1960, and PLE was recognized as a separate clinical entity in 1968.6,7 PLE is most frequently associated with lung cancer (50%), of which 80% of cases are SCLC. It has also been associated with testicular cancer (20%), breast cancer (8%), thymus cancer, and lymphoma (1-2%).8,9,10,11 It is not uncommon for non-SCLC (NSCLC) to present as a mixed component of SCLC, and it has been reported that up to one-third of patients with NSCLC present with neuroendocrine differentiation that could be the cause of a PLE.12 Intriguingly, our patient was affected by pure squamous cell carcinoma of the lung. To the best of our knowledge this is only the second such case to be described in the literature.13
The pathologic features of PLE are characterized by affected limbic structures with microscopic perivascular lymphocytic infiltration, neuronal cell loss, and reactive microglial proliferation. The current consensus is that paraneoplastic syndrome is mediated by the immune system and is secondary to cytotoxic T-cells attacking neurons. Apoptotic tumor cells are captured by tissue dendritic cells and trigger a specific T-cell-mediated response.14 The clinical symptoms typically include cognitive disorders (92%), with anterograde memory alteration, confusion, and visuospatial deficiency, and psychiatric syndromes (50%) such as depression, anxiety, and hallucinations. One or more seizures may be observed in 58% of patients, predominantly of temporal lobe origin. The neurologic signs of extralimbic damage can be observed in 42% of cases, including more diffuse brain damage, cerebellar ataxia, and peripheral neuropathy.15
MRI plays an important role in the diagnosis of limbic encephalitis. A hyperintensity is typically observed on T2-weighted images, although it can be better observed on FLAIR sequences and diffusion-weighted images, primarily in the temporal lobe (60-80%) and the limbic mesial cortical structures (i.e., the hippocampus, amygdala, hypothalamus, and insular and cingulate cortexes).5 The CSF exhibits features of inflammation in 80% of patients, with lymphocytic pleocytosis, elevated protein, oligoclonal bands, or elevated IgG. PLE is by definition associated with cancer, but it is not caused by primary or metastatic neoplasia in the nervous system. Therefore, the diagnosis of limbic encephalitis requires the search for an occult malignancy, and the specific diagnosis of PLE requires a cancer diagnosis within 5 years following the onset of PLE or the presence of a well-characterized onconeuronal antibody. Major paraneoplastic antibodies are detected in 60% of PLE cases. Anti-Hu and -ANNA-3 are predominantly associated with SCLC (94%), anti-Ma2/anti-Ta with testicular cancer, and anti-CV2/CRMP5 with lymphoma or SCLC. However, antineuronal antibodies can be undetectable in 40% of PLE cases, as observed in our patient, and their absence does not preclude the diagnosis.1,16,17 In fact, PLE with positive onconeuronal antigens typically improves after treatment of the primary tumor because the mechanism has an immune component.2
The differential diagnosis of PLE in a patient with cancer may be difficult and includes many other cancer-related complications, such brain metastases, toxic and metabolic encephalopathies, infections (especially herpes simplex encephalitis), and neurotoxic adverse effects of chemotherapy or other drugs. In addition, the diagnosis requires the exclusion of deficiency states such as Wernicke-Korsakoff syndrome, inflammatory disorders (i.e., acute disseminated encephalomyelitis), primary CNS malignancies (e.g., lymphoma), and neurodegenerative disorders (e.g., Creutzfeldt-Jakob disease).3,4
In conclusion, this is only the second report in the literature to describe PLE associated with squamous cell carcinoma of the lung. Our case presented with neurologic features and MRI alterations consistent with PLE with no detectable onconeuronal antibodies. The neurologic symptoms in our patient improved after surgery to remove the primary tumor; however, autopsy confirmed the persistence of bilateral limbic encephalitis.
Figures and Tables
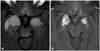 | Fig. 1Brain fluid attenuated inversion recovery imaging performed before surgery showing a diffuse hyperintense signal within the medial temporal lobes and extending into the basal ganglia, frontal basal lobes, and leptomeninges (A). Avid and homogeneous enhancement was noted after administration of contrast medium (B). |
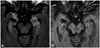 | Fig. 2Brain fluid attenuated inversion recovery imaging performed after surgery depicting remarkable decreases in the extension and signal intensity within the medial region of both temporal lobes. Secondary mesial atrophy with temporal horn dilatation can be observed (A). No enhancement was observed after gadolinium injection (B). |
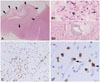 | Fig. 3Histologic images of postmortem brain tissue showing a prominent atrophy of the anterior part of the hippocampus at low magnification (A) (hematoxylin-eosin stain) with marked segmental neuronal loss and loosening of the neuropil (arrows). At higher magnification, prominent reactive gliosis with abundant large gemistocytes (B1), some mineralizations (B1), and parenchymal and perivascular inflammatory cuffs (B2) were observed. Most of the parenchymal infiltrates were composed of CD8-positive T-cells (C), some of which were in close contact with morphologically intact neurons (arrows) (D). |
Acknowledgements
Histological images were kindly provided by the Neurological Tissue Bank of the Biobanc-Hospital Clinic-Institut d'Investigacions Biomèdiques August Pi i Sunyer IDIBAPS (Dr. Ellen Gelpi).
The authors thank to Dr. Francesc Graus Ribas from the Onconeurology Unit of the Clinic University Hospital of Barcelona for his generous advices. This manuscript version has been kindly reviewed by the English Teacher Katie Linder.
References
1. Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004; 75:1135–1140.

2. Bataller L, Kleopa KA, Wu GF, Rossi JE, Rosenfeld MR, Dalmau J. Autoimmune limbic encephalitis in 39 patients: immunophenotypes and outcomes. J Neurol Neurosurg Psychiatry. 2007; 78:381–385.

4. Dirr LY, Elster AD, Donofrio PD, Smith M. Evolution of brain MRI abnormalities in limbic encephalitis. Neurology. 1990; 40:1304–1306.

5. Lawn ND, Westmoreland BF, Kiely MJ, Lennon VA, Vernino S. Clinical, magnetic resonance imaging, and electroencephalographic findings in paraneoplastic limbic encephalitis. Mayo Clin Proc. 2003; 78:1363–1368.

6. Brierley JB, Corsellis JA, Hierons R, Nevin S. Subacute encephalitis of later adult life. Mainly affecting the limbic areas. Brain. 1960; 83:357–368.

7. Corsellis JA, Goldberg GJ, Norton AR. "Limbic encephalitis" and its association with carcinoma. Brain. 1968; 91:481–496.

8. Burton GV, Bullard DE, Walther PJ, Burger PC. Paraneoplastic limbic encephalopathy with testicular carcinoma. A reversible neurologic syndrome. Cancer. 1988; 62:2248–2251.

9. Dögel D, Beuing O, Koenigsmann M, Diete S. [Paraneoplastic limbic encephalitis resulting from non-Hodgkin-lymphoma: two case reports]. Fortschr Neurol Psychiatr. 2008; 76:41–46.

10. Alamowitch S, Graus F, Uchuya M, Reñé R, Bescansa E, Delattre JY. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain. 1997; 120(Pt 6):923–928.

11. Rajappa S, Digumarti R, Immaneni SR, Parage M. Primary renal lymphoma presenting with paraneoplastic limbic encephalitis. J Clin Oncol. 2007; 25:3783–3785.

12. Howe MC, Chapman A, Kerr K, Dougal M, Anderson H, Hasleton PS. Neuroendocrine differentiation in non-small cell lung cancer and its relation to prognosis and therapy. Histopathology. 2005; 46:195–201.

13. Dabbeche C, Guyon D, Loubes-Lacroix F, Manelfe C. [Paraneoplastic limbic encephalitis associated with epidermoid lung carcinoma]. J Neuroradiol. 2005; 32:278–280.
14. Voltz R. Paraneoplastic neurological syndromes: an update on diagnosis, pathogenesis, and therapy. Lancet Neurol. 2002; 1:294–305.

15. Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000; 123(Pt 7):1481–1494.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download