Abstract
Background
Clinicians are searching for new methods to diagnose and predict the course of androgenetic alopecia noninvasively.
Objective
Our aim is to evaluate trichoscopic findings and their relations with disease severity in androgenetic alopecia.
Methods
The videodermatoscopic findings of 143 female and 63 male patients with androgenetic alopecia were compared with each other, with those of healthy subjects (n=100), and with those of patients with other nonscarring alopecias (n=208). Mann-Whitney U-test, χ2 analyses, and logistic regression analysis were used for statistical analysis.
Results
No statistically significant relation was found between trichoscopic findings and severity in male androgenetic alopecia (MAGA) on the basis of the modified Hamilton Norwood scale (among 7 degrees); however, multihair follicular unit and perifollicular pigmentation were related to low severity whereas white dots, honeycomb pattern pigmentation, and brown dots were related to high severity. On the other hand, according to the Ludwig classification, arborizing red lines were related to low severity and brown dots were related to high severity, whereas there was no difference in stages between the Ebling and Olsen classifications in female androgenetic alopecia (FAGA). In the characteristic trichoscopic findings in this study, perifollicular pigmentation was found as a normal feature of the scalp, whereas multihair follicular unit and honeycomb pigment pattern, which were previously considered as normal features, were observed to be related to androgenetic alopecia.
Androgenetic alopecia (AGA) is the most common form of hair loss both in men and women, and is characterized by a progressive loss of hair diameter, length, and pigmentation1. It is sometimes difficult to diagnose AGA clinically; thus, dermatologists are searching for new methods to facilitate the diagnosis. Trichoscopy is one of these methods, and AGA is characterized trichoscopically according to an increased proportion of thin and vellus hairs, hair shaft thickness heterogeneity, perifollicular discoloration (hyperpigmentation), and the presence of a variable number of yellow dots (YD), as reported in previous studies2,3,4,5.
This study aimed to evaluate the trichoscopic findings in AGA in relation to disease severity, as well as to compare the trichoscopic findings of male AGA (MAGA) with those of female AGA (FAGA).
The whole scalp of 206 (143 women, 63 men) patients with AGA was evaluated by using videodermoscopy in dermatology outpatient clinics between January 2011 and June 2011. This study was approved by local ethics committee. The trichoscopic findings of the 143 FAGA and 63 MAGA patients were compared with each other according to disease severity, as well as compared with those of patients with other nonscarring alopecias, including seborrheic dermatitis (n=112), alopecia areata (AA) (n=39), psoriasis (n=31), telogen effluvium (TE) (n=22), and trichotillomania (TC) (n=4), and with those of healthy subjects (n=100). Among the FAGA patients in the study group, 56 also had seborrheic dermatitis and 3 also had psoriasis. Moreover, 23 of the MAGA patients also had seborrheic dermatitis and 4 of them also had psoriasis.
FAGA was clinically suspected in cases of frontal (Christmas tree pattern), diffuse central, or vertex/frontal (male pattern) accentuation with sparing of the occipital area6. The diagnosis was established through clinical examination and confirmed with scalp biopsy in ambiguous cases. Three patients suspected of having FAGA or TE had punch biopsy specimens taken from the immediately adjacent skin on the midscalp, and all specimens were sectioned horizontally. The terminal-to-vellus hair ratio at the midisthmus level was used to set the diagnosis. A ratio of ≤4 : 1 was accepted as indicative of FAGA, whereas a ratio of <8 : 1 together with an anagen-to-telogen ratio of <8 : 1 was accepted indicative of TE7. The severity of FAGA was estimated according to Ludwig's scale in 3 degrees, and cases with frontal accentuation (Christmas tree pattern) were estimated according to the Olsen scale in 3 degrees. Patients with diffuse central or vertex/frontal (male pattern) alopecia sparing the occipital area were classified as having FAGA of a male pattern, and the severity was estimated according to Ebling's classification in 5 degrees8,9.
Similar to FAGA, the diagnosis of MAGA was established according to clinical findings. These findings were alopecia starting from the temporal and midfrontal scalp and reaching to the posterior scalp, or centrifugal alopecia starting from the vertex. In 3 patients with MAGA alopecia, the FAGA pattern was observed similar to some Asian men; there was diffuse thinning in the frontal and midline areas, whereas temporal and vertex areas were less affected. To estimate the disease severity in MAGA patients, the modified Hamilton Norwood (H-N) scale was used and alopecia was graded in 7 degrees. Afterward, the MAGA severity was categorized as follows: MAGA disease severity group 1 (DSG-1) as the sum of H-N scales 1, 2, and 3, and MAGA disease severity group 2 (DSG-2) as the sum of H-N scales 4, 5, 6, and 7.
To elucidate the vascular patterns and to avoid nosocomial infections, a solution combining propanol and butanediol and glycerin as immersion gel were used in limited cases before trichoscopic examination. At least 4 images were taken with ×100 magnification from the parietal, frontal, occipital, and lesional areas.
Statistical analysis was performed by using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). The concordance of the data to normal distribution was checked with the Kolmogorov-Smirnov test. Continuous data are given as the mean±standard deviation. Multiple logistic regression analysis tests were applied to determine the relation between variables and risk factors. Categorical variables were summarized as percentages and compared by using the χ2 test. Mann-Whitney U-test was applied to compare 2 groups with continuous data that did not show a normal distribution. Significance was set at p<0.05.
The age of the MAGA patients varied from 12 to 75 years (mean, 37.3±16.6 years), and that of the FAGA patients varied from 15 to 79 years (mean, 34.3±15.2 years). The patients enrolled in our study had mostly type III Fitzpatrick's skin phenotype.
The common trichoscopic findings were hair diameter diversity (HDD), structureless red areas (SRA), brown dots (BD), and perifollicular white scales (PWS) (p<0.001). Among the less common trichoscopic findings were white dots (WD), YD, multihair follicular unit (MHFU), and hidden hair (HH) (p<0.05; Table 1). The common trichoscopic findings in MAGA compared with those in other nonscarring alopecias are summarized in Table 1.
There was no statistically significant relation between MAGA severity (among 7 degrees in the modified H-N scale) and trichoscopic findings, although WD were seen in 5 of 9 patients in MAGA degree 5 (p=0.098). On the other hand, when the 2 MAGA severity groups (DSG-1 and DSG-2) were compared, MHFU and perifollicular pigmentation (PFP) were found to be related to low severity (mostly seen in DSG-1), whereas WD, honeycomb pigment pattern (HCPP), and BD were observed to be related to severe disease (mostly seen in DSG-2) (Table 2). The relations of trichoscopic findings to disease severity in the DSG-1 and DSG-2 are summarized in Table 2.
Among female patients in whom disease severity was assessed according to Ludwig classification, the common trichoscopic findings were miniaturization, PFP, MHFU, twisted red loops, arborizing red lines (ARL), SRA, HH, atypical red vessels (ARV), YD, WD, and BD (p<0.05; Table 3). HCPP was also frequent (13.4%, p=0.268); however, the frequency was not statistically significant as seen in MAGA.
When FAGA cases were classified according to the Ebling and Olsen classifications, there was no difference between trichoscopic findings in the different severity groups. In the Ludwig classification, ARL was detected to be more common in stage 1 and BD was detected to be more common in stage 3 when compared with other nonscarring alopecias (Table 4). Accordingly, early-stage FAGA (low disease severity) was found to be related to ARL and late-stage FAGA (high disease severity) was observed to be related to BD similar to MAGA. In the early stage (low-severity MAGA), PFP and MHFU were common; in the late stage (high-severity MAGA), HCPP, BD and WD were common. On the other hand, when the frequencies of the common trichoscopic findings were compared between MAGA and FAGA, only 2 findings were statistically more common: WD and BD in MAGA.
Similar to the findings of Ross et al.3, WD were more common in chronic and/or severe disease in our study (Fig. 1A, B).
Abraham et al.10 related WD to eccrine glands and follicular ostia histologically in their study. In AGA, the 5-α-reductase activity stimulates sebaceous glands and causes hypertrophy; however, its effect on eccrine glands is not clearly known11,12. Although it is known that the eccrine glands are stimulated mainly by cholinergic stimulation, 5-α-reductase activity could also be seen in acrosyringium, which may cause these glands to undergo hypertrophy like sebaceous glands in AGA. In our study, the WD in AGA were not similar to those of AA, and the distances between WD were detected to be more variable in AGA; thus, we thought that this scattered form of WD in AGA can be attributed to 5-α-reductase activity and its effects on both the acrosyringium and pilosebaceous ostium11.
Although AGA is classified within the nonscarring alopecias, it could also show perifollicular infiltrates as a histopathological feature. Furthermore, in advanced disease, follicles can be replaced by connective tissues, leading to fibrous tracts and finally causing atrophy in follicles. These empty follicular ostia are seen as WD3,13,14,15,16,17,18,19. In concordance with this finding, WD were observed to be more common in the late stages of AGA in our study. On the other hand, Kossard and Zagarella19 observed WD in cases with scarring alopecia and considered these WD as being the melanin pour places in fibrous tracts of scar tissue. Thus, it could be postulated that the fibrous tracts seen in the late stages of AGA can also cause these trichoscopic findings such as WD.
As dark skin color makes WD more obvious, the frequency of WD in our study was high because our patients had dark skin color, similar to the findings of Zhang et al.20. To our knowledge, interfollicular pinpoint WD can be seen in the sun-exposed scalp of patients with skin phototypes III and IV and in the normal scalp of those with phototypes V and VI10. These dots appear as small (0.2~0.3 mm) WD distributed regularly in the interfollicular scalp, dispersed across the mosaic pigmented network. They have been correlated with the acrosyringeal and follicular openings10,21. In our study, we saw both these regular pinpoint WD between the HCPP together with irregularly distributed white areas.
According to our results, WD were more common in alopecia totalis, sisaipho, and late-stage MAGA. The common features of these 3 conditions were the effect of cumulative, direct sun damage with inflammatory infiltrates. Additionally, in our opinion, the more dense and irregularly distributed form of AGA is attributed to 5-α-reductase activity in the glands.
Women usually have longer hair than men; thus, damage caused by the sun and, consequently, the frequency of HCPP are less frequent in women. Thus, in our study group, WD were observed less commonly in FAGA than in MAGA.
In our study group, HCPP was observed to be more common in severe MAGA. Ross et al.3 related HCPP to chronic sun exposure, whereas Tosti observed WD in subjects with chronic sun exposure and in those with dark skin22. Thus, to determine the effect of cumulative sun exposure on scalp trichoscopic findings, we classified our patients and 100 healthy controls into 2 groups according to their ages: ≤25 years and ≥50 years. The ratio of HCPP in the control group was 0 : 43 in the ≤25-year age group and 2 : 20 in the ≥50-year age group. Furthermore, the ratio of HCPP in patients with alopecia was 16 : 122 in the ≤25-year age group and 11 : 43 in the ≥50-year age group. The difference between the 2 age groups was found to be not significant in both the alopecia and control groups; however, HCPP was detected to be significantly more common in the alopecia group. Logistic regression analysis revealed that when this pattern is observed trichoscopically, the estimated alopecia risk was 3.2 times as high.
In our study group, HH was common in patients with FAGA, MAGA, seborrheic dermatitis, and psoriasis. The presence of psoriasiform hyperplasia and dermal infiltrates both in seborrheic dermatitis and psoriasis together cause epidermal thickness, whereas pilosebaceous unit was sparse11,12,23. Consequently, the hair shaft appeared hidden and lying under this thickened epidermis, prompting us to name this appearance as HH (Fig. 1C).
This sign, observed in AGA in our study group, was attributed to accompanying seborrheic dermatitis and psoriasis with miniaturized hair shafts in those patients. On the other hand, isolated AGA may be the only reason because dermal infiltrates are also common in AGA, which may cause epidermal augmentation similar to seborrheic dermatitis and psoriasis24.
Hair density is related to the number of hairs extending from 1 pilosebaceous unit; in a healthy person, this is about 1~3 hairs from 1 unit. Thus, Rakowska et al.4 accepted 1 hair pilosebaceous unit as a minor criterion for AGA18. We assessed regularly arranged ≥3 hairs from 1 unit without any epidermal and peripilar sign as MHFU that differs from tufted hairs seen in folliculitis decalvans, lichen planopilaris, and central centrifugal cicatricial alopecias25. Although it could be seen in both healthy persons and alopecia patients, MHFU was found to be related to alopecia in our study group. To our knowledge, the percentage of follicular units with only 1 hair is also increased in TE and various forms of anagen hair loss4,26. It was found to be statistically significant in MAGA and FAGA when compared with other alopecias and with the healthy control group in addition to this literature.
MHFU was also found to be related to less severe MAGA and FAGA (Fig. 1D). In early AGA, dermal and perifollicular inflammation constituting growth factors and cytokines, together with the anabolic effects of testosterone, may provoke this augmentation.
PFP was first described by Deloche et al.24. Inui et al.2 reported PFP in almost all patients with alopecia who had fair skin; however, the ratios were lower in Asian patients, similar to our study. PFP is thought to be the result of dermal infiltrates in AGA24,25,26,27,28,29. Perifollicular inflammation is thought to be due to the effect of cosmetics, chemicals, ultraviolet light, mucin deposit, and melanocytes; however, the etiopathogenesis is still unknown15,29.
PFP was found to be significantly more common in FAGA patients than in MAGA patients in our study group. Thus, inflammation could be postulated to be more severe in FAGA (Fig. 1A, B).
When the relation between skin color and PFP was evaluated, PFP was found to be more common in Fitzpatrick's skin phenotype 3 both in the alopecia and control groups, although it was not statistically significant. The ratio of PFP in the control group was 15 : 43 in the ≤25-year age group and 0 : 20 in the ≥50-year age group, and the frequency of PFP was not different between the alopecia and control groups. Logistic regression analysis for PFP revealed that when found trichoscopically, the estimated risk for alopecia is 3.2 times higher.
Thus, PFP seemed to be a normal hair feature in persons younger than 25 years. Deloche et al.24 detected high peripilar pigmentation in subjects having high hair density. Furthermore, Rakowska et al.4 related high hair density with the number of hairs extending from 1 follicular unit. Thus, MHFU and PFP were found to be common in our healthy young controls aged <25 years.
The ratio of YD were 16 : 63 in MAGA and 25 : 143 in FAGA in our study, similar to previous studies: 13 : 50 in Deloche et al.'s study24, 6 : 34 in MAGA and 1 : 7 in FAGA in Inui et al.'s study30, and 13 : 50 in MAGA and 1 : 10 in FAGA in Inui et al.'s other study2. YD were also seen in TC (1 : 4), psoriasis (6 : 31), seborrheic dermatitis (44 : 112), and TE (1 : 22) in our study group, whereas Rakowska et al.4 described YD as the main dermoscopic criteria to discriminate FAGA and chronic TE.
YD in AGA are thought to be the result of sebaceous hypertrophy and lagooning in glands as a result of end-organ hypersensitivity4. YD were observed to be more common in MAGA in both Inui et al.'s2,30 study groups and ours in relation to higher levels of androgens supporting this hypothesis.
YD are fırst described as uniform pink-YD by Ross et al.3; however, in our study, some of them were brown. BD were detected to be a marker of severity both in MAGA and FAGA in our study (Fig. 1A).
Rudnicka et al.31,32 described scattered brown areas in discoid lupus erythematosus and actinic keratosis and regularly distributed gray or brown-gray dots in the eyebrow area of patients with frontal fibrosing alopecia. In addition, Fu et al.33 identified dirty dots as a normal finding in the scalps of 10 of 19 healthy children that represent nonmicrobial environmental particles. On the other hand, in our study, we saw BD in AGA located in perifollicular and interfollicular areas similar to YD that we think are brown because of the dark skin color of the patients.
The characteristic trichoscopic findings of AGA are known as HDD and PFP34. Similar to previous studies, all of our patients with AGA demonstrated HDD, confirming that this sign is the main criterion in AGA2,34,35 (Fig. 1A~D).
There are no data about the relation between MAGA severity and trichoscopic findings between classifications such as Ludwig, Ebling, Olsen, and modified H-N. On the other hand, Zhang et al.20 investigated the relation between FAGA and trichoscopic findings according to the Ludwig classification. In their study, the characteristic trichoscopic features (at 10-fold magnification) of FAGA were brown and white peripilar signs, WD, scalp pigmentation, and focal atrichia. White peripilar signs, scalp pigmentation, and focal atrichia positively correlated with the stage (Ludwig stage 3)20. In both Zhang et al.'s20 and our study, it would be more appropriate to use the basic and specific (BASP) classification in addition to Ludwig, Ebling, Olsen, and modified H-N scale that is applicable to both MAGA and FAGA. The BASP classification is a new stepwise, systematic, and universal system for patterned hair loss, regardless of sex36.
There is no study in the literature about the relation between MAGA severity and trichoscopic findings according to different disease severity classifications. On the other hand, Ross et al.3 emphasized YD being higher in late AGA in their study. In addition, Lacarrubba et al.5 underlined miniaturization as being higher in early AGA.
Besides, in our study, PFP was detected to be a characteristic trichoscopic finding of AGA that was described as a normal feature of the scalp in healthy persons younger than 25 years. In addition, MHFU and HCPP, which were previously accepted as normal features, were also found to be related to alopecias in this study.
Among the study group patients with FAGA, 56 also had seborrheic dermatitis and 3 also had psoriasis; thus, trichoscopic features such as ARL, ARV, and SRA were attributed to the accompanying diseases in these patients.
Twenty-three of the MAGA patients also had seborrheic dermatitis and 4 of them also had psoriasis; thus, trichoscopic figures such as PWS, SRA, signet ring vessel, and HH were attributed to additional diseases. Similar to our findings, Ross et al.3 detected red loops in AGA patients and ARL, HCPP, and YD in patients having both AGA and seborrheic dermatitis. On the other hand, on patient selection, it might be more accurate to select AGA patients without seborrheic dermatitis, or compare groups with or without seborrheic dermatitis.
To have more meaningful and significant results, a better-designed study with a larger number of patients and controls should be done in different age groups and the results confirmed histopathologically.
Figures and Tables
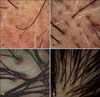 | Fig. 1(A) Perifollicular pigmentation, brown dots, white dots, miniaturization, and hair diameter diversity in a 65-year-old patient with male androgenetic alopecia Hamilton Norwood stage 5. (B) Miniaturization, hair diameter diversity, white dots, and perifollicular pigmentation in a 25-year-old patient with female androgenetic alopecia (FAGA) Ludwig stage 1. (C) Perifollicular pigmentation, glomerular, and signet ring vessel (in green circle), hidden hairs, miniaturization, and hair diameter diversity in a 23-year-old FAGA Ludwig stage 1 patient with seborrheic dermatitis. (D) Perifollicular pigmentation, multihair follicular unit, miniaturization, and hair diameter diversity in a 32-year-old FAGA Ludwig stage 1 patient. |
Table 1
Common trichoscopic findings in patients with male androgenetic alopecia in comparison with patients with other nonscarring alopecias (N=63)

References
1. Gordon KA, Tosti A. Alopecia: evaluation and treatment. Clin Cosmet Investig Dermatol. 2011; 4:101–106.

2. Inui S, Nakajima T, Itami S. Scalp dermoscopy of androgenetic alopecia in Asian people. J Dermatol. 2009; 36:82–85.

3. Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006; 55:799–806.

4. Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia: method standardization and diagnostic criteria. Int J Trichology. 2009; 1:123–130.

5. Lacarrubba F, Dall'Oglio F, Rita Nasca M, Micali G. Videodermatoscopy enhances diagnostic capability in some forms of hair loss. Am J Clin Dermatol. 2004; 5:205–208.

9. Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977; 97:247–254.

10. Abraham LS, Piñeiro-Maceira J, Duque-Estrada B, Barcaui CB, Sodré CT. Pinpoint white dots in the scalp: dermoscopic and histopathologic correlation. J Am Acad Dermatol. 2010; 63:721–722.

11. Wolff H. Diseases of hair. In : Braun-Falco O, Plewig G, Wolff HH, Landthaler M, editors. Braun-Falco's Dermatology. 3th ed. Heidelberg: Springer Verlag;2009. p. 1029–1059.
12. Paus R, Peker S, Sundberg JP. Biology of hair and nails. In : Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. 2th ed. St. Louis: Mosby Elsevier;2008. p. 965–987.
13. Whiting DA. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J Am Acad Dermatol. 1993; 28:755–763.

14. Whiting D. Scalp biopsy as a diagnostic and prognostic tool in androgenic alopecia. Dermatol Ther. 1998; 8:24–33.
15. Won CH, Kwon OS, Kim YK, Kang YJ, Kim BJ, Choi CW, et al. Dermal fibrosis in male pattern hair loss: a suggestive implication of mast cells. Arch Dermatol Res. 2008; 300:147–152.

16. Bertolino PA, Freedberg IM. Disorders of epidermal appendages and related disorders. In : Freedberg IM, Eisen AZ, Wollf K, editors. Dermatology in general medicine. 4th ed. New York: McGraw-Hill;1993.
17. Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Dermatol Surg Oncol. 1990; 16:1127–1133.

18. Leroy T, Van Neste D. Contrast enhanced phototrichogram pinpoints scalp hair changes in androgen sensitive areas of male androgenetic alopecia. Skin Res Technol. 2002; 8:106–111.

19. Kossard S, Zagarella S. Spotted cicatricial alopecia in dark skin. A dermoscopic clue to fibrous tracts. Australas J Dermatol. 1993; 34:49–51.
20. Zhang X, Caulloo S, Zhao Y, Zhang B, Cai Z, Yang J. Female pattern hair loss: clinico-laboratory findings and trichoscopy depending on disease severity. Int J Trichology. 2012; 4:23–28.

21. Ardigò M, Tosti A, Cameli N, Vincenzi C, Misciali C, Berardesca E. Reflectance confocal microscopy of the yellow dot pattern in alopecia areata. Arch Dermatol. 2011; 147:61–64.

22. Tosti A. Alopecia areata. In : Tosti A, editor. Dermoscopy of hair and scalp disorders with clinical and pathological correlations. London: Informa Healthcare;2007. p. 26–50.
23. Cotsarelis G, Botchkarev V. Biology of hair follicles. In : Wollf K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7th ed. New York: McGraw-Hill;2008. p. 39–749.
24. Deloche C, de Lacharrière O, Misciali C, Piraccini BM, Vincenzi C, Bastien P, et al. Histological features of peripilar signs associated with androgenetic alopecia. Arch Dermatol Res. 2004; 295:422–428.

26. Rakowska A. Trichoscopy (hair and scalp videodermoscopy) in the healthy female. Method standardization and norms for measurable parameters. J Dermatol Case Rep. 2009; 3:14–19.

27. Young JW, Conte ET, Leavitt ML, Nafz MA, Schroeter AL. Cutaneous immunopathology of androgenetic alopecia. J Am Osteopath Assoc. 1991; 91:765–771.

28. Headington JT. Transverse microscopic anatomy of the human scalp. A basis for a morphometric approach to disorders of the hair follicle. Arch Dermatol. 1984; 120:449–456.

29. Jaworsky C, Kligman AM, Murphy GF. Characterization of inflammatory infiltrates in male pattern alopecia: implications for pathogenesis. Br J Dermatol. 1992; 127:239–246.

30. Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: analysis of 300 cases. Int J Dermatol. 2008; 47:688–693.

31. Rudnicka L, Rakowska A, Olszewska M. Trichoscopy: how it may help the clinician. Dermatol Clin. 2013; 31:29–41.
32. Rudnicka L, Olszewska M, Rakowska A. Atlas of trichoscopy: dermoscopy in hair and scalp disease. London: New York;Springer-Verlag. p. 2012.
33. Fu JM, Starace M, Tosti A. A new dermoscopic finding in healthy children. Arch Dermatol. 2009; 145:596–597.

34. Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011; 38:71–75.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download