This article has been
cited by other articles in ScienceCentral.
Abstract
The distribution of caveolin isoforms was previously evaluated in the retinas of different species, but has not yet been described in the primate retina. In this study, the distribution of caveolins was assessed via immunochemistry using isoform-specific antibodies in the retina of the black-and-white ruffed lemur. Here, we report the presence of a variety of caveolin isoforms in many layers of the lemur retina. As normal human retinas were not available for research and the retinas of primates are fairly similar to those of humans, the lemur retina can be utilized as a model for caveolin distribution in normal humans.
Keywords: caveolin, immunocytochemistry, lemur, retina
Caveolins are integral membrane proteins which are principal components of the omega-shaped plasma membrane invaginations referred to as caveolae. Multiple forms of caveolin have been identified thus far, and have been designated caveolin-1, caveolin-2, and caveolin-3. These variants differ with regard to specific properties and tissue distribution. Caveolin-1 and caveolin-2 may originate from a common ancestor, and are expressed most abundantly in adipocytes, endothelial cells, fibroblasts, and smooth muscle cells [
7]. They have also been identified in neuronal cells [
2]. Caveolin-3 expression was previously believed to be muscle-specific [
7], although it has been shown to be present in astroglial cells [
6] and neurons of the vegetative ganglions as well [
5]. Scherer et al. [
8] also previously identified two caveolin isoforms within
Caenorhabditis elegans, demonstrating that caveolins are both structurally and functionally conserved across species from worms to humans. These data indicate that caveolins may play an important evolutionary role. The distribution of caveolin isoforms was studied previously in the retinas of different species. As data regarding the primate retina had not yet been compiled, we elected to study the retina of the blackand- white ruffed lemur (
Varecia variegata variegata).
The male lemur utilized in this study lived in captivity and died naturally at the age of 11 (normally these animals live for 19 years), without any eye diseases. Within a few hours of the death of the lemur, the bulb was removed and subsequently placed in a fixative (4% paraformaldehyde), then incubated for 24 h at 4℃. The retina was carefully detached from the posterior eyecup. The retinas were then incubated overnight in 30% sucrose. Three samples were obtained from the lemur retina following a radial plane, and including the macular region, periphery, and ciliary body. 10 µm-thick radial sections were prepared on a Shandon cryotome (Thermo Scientific, USA). The distribution of the caveolins was determined via immunochemistry using isoform-specific antibodies. The primary antibodies anti-caveolin-1 (1 : 100, polyclonal rabbit IgG; BD Biosciences, USA), anti-caveolin-2 (1 : 200, monoclonal mouse IgG; BD Biosciences, USA), and anti-caveolin-3 (1 : 100, monoclonal mouse IgG; BD Biosciences, USA) were diluted in 1% BSA and incubated overnight at 4℃. In order to detect caveolin-1 and -3, anti-rabbit and anti-mouse Alexa Fluor 488 (Invitrogen, USA), respectively, were employed as secondary antibodies. As caveolin-2 yielded a weak signal that was difficult to detect, we utilized biotinylated anti-mouse, then Streptavidin Alexa Fluor 488 (Invitrogen, USA), 1 : 100, in an effort to amplify the signal. Even when this extra method was used, caveolin-2 yielded the weakest signal among the variants. In order to visualize the cytoskeleton, Alexa Fluor 594-labeled phalloidin (Invitrogen, USA) diluted to 1 : 100 was utilized to stain the F-actin. The slide was covered with 4,6-diamidino-2-phenylindole (Vectashield HardSet Mounting Medium; Vector, USA). Control reactions were conducted using rabbit and mouse normal serum with non-specific primary antibodies, and the primary antibodies were omitted in order to prevent non-specific binding. Fluorescent triple-labeled specimens were inspected on a 2100 Multi Photon Imaging System (Radiance, USA) coupled to an Eclipse E800 microscope using a LaserSharp 2000 (Nikon, USA). Adobe Photoshop 7.0 and Confocal Assistant were used for primary image processing.
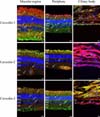
Caveolin-1: In the macular region, caveolin-1 was detected in every layer. Among these layers, the density of immunolabeling evidenced only slight differences. Both the outer and inner segments harbored caveolin-1. In the outer and inner nuclear layers, caveolin-1 was localized within the cell membranes. The ganglion layer and the outer and inner plexiform layers diffusely expressed caveolin-1. In the periphery, caveolin-1 was localized in the same layers, but at lower densities. In the ciliary body, both layers harbored caveolin-1. The immunoreactivity was weaker in the inner layer (
Fig. 1,
Table 1).
Caveolin-2: The immunostaining of caveolin-2 differed from that of caveolin-1. The lemur retina barely evidenced any caveolin-2 signals. Weak immunoreactivity was detected within the ganglion layer. The label was somewhat stronger around the blood vessels, including the vessel cells and blood cells. No signal was detected in the ciliary body samples (
Fig. 1,
Table 1).
Caveolin-3: In the macular region and the periphery, the immunostaining pattern of caveolin-3 was similar to that of caveolin-1, but the density was significantly lower. Labeling was detected only between the ganglion cell layer and the inner limiting membrane. Immunostaining densities ranged from low to moderate. Caveolin-3 was also detected within the ciliary body (
Fig. 1,
Table 1).
A summary of immunostaining densities in the various tissue samples is provided in
Table 1.
Only a few reports have been published thus far regarding the presence and distribution of caveolin in the retina. Caveolin-1 has been detected within the outer plexiform layer of the mouse retina at the synaptic ribbon in the photoreceptor terminals [
3]. In another study, caveolin-1 was detected in various layers of the rat retina, from the inner plexiform layer to the outer limiting membrane. This suggests that caveolin-1 is expressed in the Müller cells. Using specific markers, it was verified that Müller cells do, indeed, harbor caveolin [
9]. Caveolin-1 was also detected in pigment epithelial cells. Laser scanning confocal microscopic analysis of intact retinal pigment epithelium showed that caveolin-1 was localized in the apical and basal surfaces [
1]. Kim et al. [
4] have reported that, in the rat retina, caveolin-1 is present in most of the retinal layers. Caveolin-2 was detected principally around the blood vessels, but it stained a few other elements as well. Only central regions of the retina were involved in this study. No examinations were conducted regarding caveolin-3. In this study, we report on the presence of a variety of caveolin isoforms in many layers of the lemur retina; however, the distribution patterns proved to be different among individual layers. Centro-peripheral differences have also been detected. As caveolins are highly conserved proteins, tissue specificity is probably similar in closely related species. We surmise that the primate retina is fairly similar to the human retina. As normal human retinas are not available research, the lemur retina can be used as a suitable model for studies of caveolin distribution in the human retina.
In conclusion, caveolin isoforms appear to be inherent components of the retinas of vertebrates. Despite their interspecies differences, it is worth studying the distribution of these proteins across the retinal layers, in order to determine their possible functions. It has been proposed that members of the caveolin family operate as scaffolding proteins. They organize and concentrate lipids, lipid-modified signaling molecules, and G proteins within the caveolae. Binding may suppress or inhibit enzyme activity via the caveolin scaffolding domain [
7]. According to previous observations as well as the results presented herein, caveolins tend to be localized within a wide range of retinal cells in different species. They appear to play an important role in the regulation of signal transduction in the retina.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download