Abstract
Foot-and-mouth disease is one of the most important viral diseases of cloven-hoofed animals. Mass vaccination is an effective method to control the disease and is frequently utilized in endemic regions. Sufficient protection of young animals is important in mass vaccination campaigns. Maternal antibodies negatively affect the success of vaccination. Hence, determination of the optimal vaccination age is crucial for the uninterrupted protection of young animals. This study was performed to identify the effect of vaccine potency and booster administration on serum neutralizing antibody titers of calves with different levels of maternal antibodies. Calves (n = 111) on a state farm were used in this study. Oil adjuvant foot-and-mouth disease vaccines with 3 PD50 and 6 PD50 potencies were used with or without booster administration. Serum samples were collected each month up to day 120 postvaccination. Virus neutralization tests were used to measure the serum neutralizing antibody titers and estimate the protection period by using pre-determined cut-off values for protection. The results revealed that a vaccination with a 6 PD50 potency vaccine, preferably followed by a booster dose, should be used to overcome maternal immunity for incessant protection.
Foot-and-mouth disease (FMD) is one of the most important viral diseases of cloven-hoofed animals. The causative agent is in the Aphthovirus genus, a member of the picornavirus family. Mortality from FMD is high in young animals due to the affinity of the virus for myocardial tissue [24]. In addition, great economic losses occur due to trade restrictions in and with countries where the disease is present. Mass vaccination is an effective method to control FMD and is frequently utilized in endemic regions [15].
Sufficient protection of young animals is an important issue in mass vaccination campaigns [19] given that susceptible young animals can aggravate outbreaks [9]. In cattle, passive transfer of antibodies from dam to neonate is possible via colostrum, which is crucial for protection against many calf diseases. The dam's age affects the amount and quality of colostrum and thereby the antibody titer circulating in calf serum [7].
An inadequate immune response in young animals in many livestock species (pigs, cattle, and horses) has been reported [20]. Some immune response-related problems are associated with the vaccination of neonates, including the neonate's immature immune system and the presence of maternal antibodies transferred from the dam [320]. Moreover, frequent vaccinations may lead to tolerance to antigens, and a high level of cortisones can negatively affect immunization responses in neonatal calves [3].
The presence of maternal antibodies decreases or may completely disappear after immunization of neonates. This outcome is observed with all types of vaccines, and there are numerous reports [181023] demonstrating that the presence of maternal antibodies affects immunization responses negatively. In theory, a vaccine should be administered after maternal antibodies have completely disappeared [20]. Many factors are involved in the duration of the presence of maternal antibodies in neonates. It is difficult to predict when maternal antibody disappearance is complete; therefore, measurement of antibody level in a neonate is necessary. However, in practice, it is difficult to measure the antibody level of each individual. Hence, a prime-boost vaccination strategy is used to overcome maternal antibody interference [5202224]. An oil adjuvant vaccine can overcome maternal interference via a single immunization [21]. The same study suggested that vaccines with an aluminum hydroxide adjuvant cannot overcome maternal immunity [21].
According to European Pharmacopeia [4], the minimum protective level of an FMD vaccine cannot be less than 3 PD50. In addition, vaccines with a 6 PD50 protective level can be used in emergency situations. When a double emulsion vaccine of 3 PD50 potency is used in a vaccination campaign, 71% of the vaccinated livestock population are protected, whereas vaccination with the same vaccine but with a 6 PD50 potency confers 81% protection [14]. In a vaccine efficacy study, a single dose of immunization can only prevent 69% of clinical FMD and 83% of severe symptoms [15]. Vaccination campaigns with > 3 PD50 oil adjuvant vaccines have been used in Turkey to combat FMD, but recently, that strategy was replaced with one that uses a 6 PD50 vaccine.
The minimum age of animals to be vaccinated during a vaccination campaign, the potency of the vaccine, and the booster dose necessary to overcome maternal antibody interference have yet to be determined. The aim of this study was to investigate the antibody response of calves with various levels of maternal antibodies to single and booster immunizations with FMD vaccines of different potencies.
Two different batches of commercial Turvac-oil FMD trivalent vaccine of 3 PD50 and 6 PD50 potencies for type A virus, produced by the SAP Institute, Turkey, were used in this study. Montanide ISA 206, (Seppic, France) oil adjuvant was used to prepare double emulsions (water-in-oil-in-water). The O TUR 07, ATUR 04/06 (A IRAN 2005), and ASIA-1TUR 11 inactivated virus cultures were used as antigens. The 3 PD50 potency vaccine contained 4 µg of type A antigen per dose, whereas the 6 PD50 potency vaccine contained 8.4 µg of type A antigen per dose. Both vaccines also contained 5 µg of type O antigens (3 PD50) and 3 µg of ASIA-1 antigens (3 PD50) per dose. The potencies of the vaccines were previously determined by vaccination-animal challenge experiments described in the 2011 World Organisation for Animal Health (OIE) manual [27]. The antibody cut-off values were set for provision of complete protection against 10,000 homologous infectious virus particles. In addition, 1.35 log10 and higher neutralizing antibody titer values were considered protective according to previous PD50 determination experiments with the ATUR 04/06 strain (data not shown).
One hundred eleven calves owned by a state farm in Konya/Turkey were used in the study. The dams had been vaccinated periodically (at four-month intervals) against FMD several times before the study started. The calves were kept together in paddocks on the farm as a beef herd under regular management practices. All calves received pooled colostrum from recent dams during their first three days. The calves were weaned at 2.5 months of age. The ages of the calves ranged from 64 to 153 days at the time of the primary vaccination. Calf breeds were Holstein-Friesian and Swiss Brown. The study was undertaken in accordance with the International Harmonization of Animal Care and Use guidelines. The study protocol was approved by the ethics committee of the SAP Institute (Ankara, Turkey) (approval No. 2016-03).
Initially, animals were randomly assigned to four groups. Each group consisted of 25 to 29 animals. Groups A and B received 3 PD50 potency vaccines, whereas groups C and D received 6 PD50 vaccines. Additionally, five unvaccinated animals were used as a maternal antibody control group and used to monitor the natural decay of the maternal antibodies. Before vaccine administration, blood samples were obtained from the caudal vein by using sterile vacuum tubes and needles. Two milliliters of vaccine was injected intramuscularly into the hind leg of each animal. Twenty-eight days after the first vaccination, groups B and D received booster doses of the initial vaccine. Blood samples were taken on day 0 and additional blood samples were obtained on days 28, 60, 90, and 120 postvaccination (pv). Sera were stored at −20℃ until the time of analysis. Based on the neutralizing maternal antibody titers on day 0, the initial four groups were reorganized to form a total of 12 sub-groups to evaluate the effect of the maternal antibody level on the response to vaccination (Table 1).
The virus neutralization test was performed as described in the 2011 OIE manual [27]. Briefly, two-fold serial dilutions of heat-inactivated serum samples from 1:4 to 1:512 were prepared by using Glasgow cell culture medium (Biochrome; Merck, Germany) in a microplate. Subsequently, equal amounts of a 100 TCID50 ATUR 04/06 working virus suspension was added to each well. After one hour of incubation at 37℃, a suspension of 6 × 105 BHK-21 cells/mL was added in equal volumes to each well. After incubation at 37oC for 72 h, the wells were observed under a microscope to observe cytopathic effects, as characterized by the presence of cell lysis. The neutralizing antibody titer was expressed as the log10 serum dilution that neutralized 50% of the virus. The assay's validity is periodically assessed by inter-laboratory validation tests among European laboratories working with the FMD virus.
Geometric mean titer values of the five animals in the unvaccinated group were calculated. To quantify maternal antibody decay, which is a process similar to a biochemical reaction, the natural logarithm (Ln) values of the mean antibody titers of the unvaccinated animals were plotted against time (days). Given the linear relationship, the decay reaction was illustrated to conform to 1st order reaction kinetics. Hence, the mean half-life of maternal antibodies was calculated from the slope of the linear graphic. The following formula was used: where t1/2 is the half-life, k is the slope of the linear curve or the reaction coefficient with units in day−1, and Ln is the natural logarithm. Another decay curve was drawn (panel B in Fig. 1) to show the geometric mean of the neutralizing antibody titer values of the animals grouped by ten-day intervals (such as 60–70, 70–80, etc.) from the first sampling day to reveal the relationship between age at first sampling and maternal antibody level.
SPSS software for Windows (ver. 19; IBM, USA) was used for statistical analysis of the data. Analysis of variance (ANOVA) was used to examine differences between samplings. Tukey's test was used to compare group data. Differences were considered significant if the p value was less than 0.05.
Panel A in Fig. 1 illustrates the distribution of the neutralizing antibody titer values of calves in their first sampling and shows that several calves were carrying maternal antibodies at first sampling. The maternal antibody titers of the unvaccinated group gradually decreased and the decay reaction of the maternal antibodies conformed to 1st order reaction kinetics. The calculated mean half-life (t1/2) of the maternal antibodies was 25.4 days according to the formula in the “Kinetics of maternal antibody decay” section. The linear relationship between titer level and time (days) is presented in panel C in Fig. 1. The R2 for that relationship was 0.9993, and the reaction coefficient was 0.0273 day−1. The slope of the geometric mean of the neutralizing antibody results for the first calf sampling period revealed that the maternal antibody titers of the calves dropped to a critical level of protection approximately 110 days after birth (panel B in Fig. 1).
After primary vaccination, a decrease in antibody levels was observed in all groups vaccinated with 3 PD50 vaccine. Initially, for the group with the highest antibody level (> 1.80 log10) vaccinated with a single dose of 3 PD50 vaccine, the arithmetic mean antibody titer was 2.24 log10. That value reduced to 1.49 log10 on day 28 after primary vaccination and decreased to a level below the protective level at day 90 pv (panel C in Fig. 2). The group with an initial low antibody level (< 1.50 log10) responded slightly to vaccination with the single dose of 3 PD50 vaccine, but the group's antibody levels remained below the protective level throughout the period of study (panel A in Fig. 2). The mean virus neutralizing (VN) antibody level of group 1 on day 60 pv was significantly lower than that in all groups (groups 2, 3, and 4) with same initial VN antibody level (p < 0.05). There was no statistically significant difference between the mean VN antibody level of groups 5 and 6 (p < 0.05).
The initial arithmetic mean antibody titer was for the group with the highest antibody level (> 1.80 log10) was 2.26 log10. After primary vaccination with 3 PD50 vaccine, this value reduced to 1.47 log10 at day 28 pv, at which time a booster dose was administered. One month following the booster dose, the titer increased to 1.84 log10 (panel F in Fig. 2). In the low initial antibody level group (< 1.50 log10), the highest antibody titer was 2.02 log10 one month after booster vaccination (panel D in Fig. 2), which was the highest level obtained by any application of the 3 PD50 vaccine. Although the high antibody group vaccinated with the 3 PD50 booster conferred protection from the beginning, the subsequent antibody level was reduced below the protective level before day 120 pv (panel E in Fig. 2). The antibody response level of the medium level maternal antibody group (1.5 to 1.8 log10) vaccinated with the 3 PD50 booster was below that of the high maternal antibody group, and the mean titers in the medium level group remained higher than the protective threshold throughout the study (panel E in Fig. 2).
After primary vaccination, a decrease in antibody levels was observed in all groups vaccinated with the 6 PD50 vaccines. The initial arithmetic mean antibody titers was 2.11 log10 for the group with the highest antibody level (> 1.80 log10). After vaccination with the 6 PD50 vaccine, this titer was reduced to 1.52 log10 (panel C in Fig. 3). The initial arithmetic mean value of the maternal antibodies in the low antibody group (< 1.50 log10) was 0.95 log10. After primary vaccination, the mean antibody titer was 0.86 log10. Subsequently, the mean titer increased to 1.81 log10 on day 60 pv and was reduced to 1.38 log10 at four months pv. A protection gap was noted in this group prior to day 60 pv (panel A in Fig. 3). There were no significant differences in VN antibody titers between the 3 PD50 booster groups (groups 2, 6, and 10) and the 6 PD50 single dose groups (groups 3, 7, and 11) at day 60 pv (p < 0.05).
In the group with the highest antibody level (> 1.80 log10), the initial geometric mean antibody titer was 2.15 log10. After primary vaccination with the 6 PD50 vaccine, this titer was reduced to 1.25 log10 at day 28 pv. Booster administration of the 6 PD50 vaccine at day 28 pv increased the titer to 2.19 log10 on day 60 pv (panel F in Fig. 3). The initial arithmetic mean maternal antibody level in the low antibody group (< 1.50 log10) was 1.15 log10. After primary vaccination, the mean antibody titer was 0.82 log10, which was the lowest value observed in this study (panel D in Fig. 3). The highest antibody response of the whole study (2.48 log10 on day 60 pv) was also obtained in this low maternal antibody group (panel D in Fig. 3). All groups that received the booster exhibited a strong response that started one month after booster administration and persisted for more than four months above the protective level (panels D, E, and F in Fig. 3). The mean VN antibody levels of groups 4 and 8 on day 60 pv were significantly higher than those in all groups administered with a single 3 PD50 dose (groups 1, 5, and 9) (p < 0.05). The mean VN antibody level of group 12 on day 60 pv was significantly higher than those in groups 1 and 5 (p < 0.05).
Vaccine is an important weapon in the fight against FMD in endemic countries. Unfortunately, vaccination with present-day, conventional vaccines provides short-term, serotype-specific protection [12]. In addition, protection from FMD is mainly dependent on vaccine effectiveness because other measures, such as animal movement restrictions or biosecurity exercises, are difficult to apply in many countries including Turkey [16]. Vaccination of young cattle provides ambiguous results as the time of vaccination, maternal antibody level, breed of animal, and adjuvants used may affect the antibody response [13].
This study was performed in calves of various ages and with varying antibody titers in a farm implementing periodic FMD vaccinations to mimic field conditions. Two different FMD vaccine potencies were investigated in calves with various levels of maternal antibodies. Additionally, the effect of booster dose administration was evaluated for both vaccine potencies. The neutralizing antibody responses of calves with ages ranging from 64 days to 153 days prior to primary vaccination and with or without booster were measured. Various levels of maternal antibodies were detected in calves born to regularly vaccinated dams. Because the dams' ages were heterogeneous hence each individual might have received different number of FMD vaccinations prior to the study.
Determination of the half-life of maternal antibodies is important because it enables estimation of the duration of protection [18]. Previous studies revealed that the average half-life of maternal antibodies was approximately 21 to 22 days [61925]. Murphy et al. [18] reported that the half-life of IgG is 28.5 days in colostrum-fed calves. In this study, the mean half-life of the maternal antibody was 25.4 days. Our result is lower than those of Murphy et al. [18] but greater than those of Nicholls et al. [19] (22 days), Späth et al. [25] (21 days), and Dekker et al. [6] (21 days). The differences among these results might be attributed to the methods used to determine half-life, as was previously suggested by Murphy et al. [18].
The virus neutralization assay is an ideal method for investigating maternal interference [2]. It has been hypothesized that B cell epitopes are masked by a high level of neutralizing antibodies. However, monoclonal antibody experiments have revealed that the mechanism is more complex, and that steric hindrance may be involved [20]. Calves with high maternal antibody levels respond to primary and secondary vaccinations with low neutralizing antibody levels, and this is independent of the total antibody response, which includes antibodies other than the neutralizing antibodies [2]. Although the presence of maternal antibodies inhibits the primary humoral response to the vaccine, B cells are able to maintain their other functional capabilities, such as differentiation to memory cells [11]. That trait can be exploited to overcome maternal interference. A booster dose can invoke memory B cells, and there are many examples of this in practice [51726]. In this study, booster administration showed its effect, particularly in the 3 PD50 group in the presence of high and low antibody influence. Moreover, the highest titers observed in this study were obtained after a 6 PD50 vaccine booster application.
One of the most important aspects of the antibody response in neonates is the ratio of antigen concentration to maternal antibody concentration [22]. Based on our results, the antigen amount per dose, as well as the provision of a booster dose, were important parameters in overcoming maternal antibody interference. In addition, the results revealed that it is impossible to obtain a robust antibody response with a 3 PD50 vaccine, even with a low maternal antibody titer, with only a primary vaccination. This situation may be explained either by the presence of insufficient antigen in the 3 PD50 vaccine for the immature immune system of young animals or the low antibody titers may not be low enough to overcome the interference. The latter is concordant with another study [24], which postulated that even trace amounts of maternal antibodies might block a strong response to FMD vaccine. Späth et al. [25] reported a similar situation with a commercial vaccine that passed potency tests in adult animals but failed to elicit strong total antibody titers, as detected with liquid phase-blocking ELISA, in calves. In the current study, as expected, the highest antibody level was obtained for the low maternal antibody level group that received primary and booster vaccines of 6 PD50 potency. Normally, it is expected that high antibody levels would suppress at least the primary antibody response and the protective period would be short. But, interestingly, the longevities of the estimated protection of the groups with high maternal antibody levels were better than those in the medium and low maternal antibody level groups for both the 3 and 6 PD50 vaccines. Similar results were obtained by Lee et al. [17] in piglets. Those authors suggested that the better response in smaller piglets might be a result of an overdose effect, given that smaller piglets receive more antigens per kilogram. As illustrated in panel A in Fig. 1, younger calves tended to have higher levels of maternal antibodies than older calves. This tendency could be due to the comparatively low weight of the younger group, as suggested by Lee et al. [17]. According to our study results, the immune response to a single dose 6 PD50 vaccine in the high-level maternal antibody group provided antibody titers greater than the protective level and for a long period of time. On the other hand, the protective periods were short for groups with initial low and medium maternal antibody levels. Therefore, it can be said that in high levels of maternal antibodies, which may be suppressed slightly by a high potency vaccine, the waning of maternal antibodies will take a long time and will remain greater than the protective level. Similarly, Späth et al. [25] reported a slight decrease at day 15 pv. The same authors observed a strong antibody response that persisted for four months in animals 3 to 4 months of age with low maternal antibody levels. In this study, the lowest values on day 28 pv were obtained in the low maternal antibody groups. At day 28 pv, all vaccinated groups had fewer neutralizing antibodies compared with the unvaccinated group. However, at day 60 pv, a significant difference in the neutralizing titers from the maternal antibody group was demonstrated for all groups except the 3 PD50 single dose group.
Comparing the 6 PD50 single and the 3 PD50 booster groups, there was a similar response pattern in the groups with high and low levels of initial maternal antibodies. However, in the medium level group, a slightly better response was obtained for the single 6 PD50 compared with vaccinations with 3 PD50 vaccines and with booster administration. According to our results, > 6 PD50 vaccines can be used not only in emergency situations but also for immunization of calves to overcome maternal interference.
Many animals carry maternal antibodies against FMD in their first few months of life in countries where mass vaccination is practiced and the disease displays an endemic character. Young animals can negatively influence herd immunity due to maternal antibody interference and vaccines with insufficient potency. Vaccines with high potency induce better immunity in dams, and more antibodies can then be transferred by colostrum. Despite interference, vaccination with a high potency vaccine, including a booster dose in the presence of a high level of neutralizing maternal antibodies, resulted in uninterrupted immunity in the calves in this study. Although it is recommended to administer a booster dose one month after primary vaccination in calves, it is not always possible for veterinarians to visit the same farm a second time in one month. Hence, dams should be vaccinated in late pregnancy. In addition, high potency vaccines should be used for immunization of calves older than 3 months of age, and, if possible, a high potency booster dose should be administered for continuous protection.
Figures and Tables
 | Fig. 1(A) Individual serum virus neutralizing antibody titers of calves according to calf age on day 0 of the experiment. Horizontal red line indicates the cut-off level of antibody titers for estimated protection. (B) Geometric means of neutralizing antibodies for calves grouped by 10-day intervals based on calf age at day 0 of the experiment. A linear slope was drawn using these titer values. Horizontal red line indicates the cut-off level of antibody titers for estimated protection. Green color filled area indicates the estimated protection period. (C) Geometric means of neutralizing maternal antibody levels of unvaccinated group. A linear slope was drawn with these titer values. Horizontal red lines indicate the cut-off level of antibody titers for estimated protection. Green color filled area indicates the estimated protection period. |
 | Fig. 2Arithmetic means of serum virus neutralizing antibody titers of calves vaccinated with 3 PD50 vaccine. (A) 3 PD50 single < 1.50, (B) 3 PD50 single 1.50–1.80, (C) 3 PD50 single > 1.80, (D) 3 PD50 booster < 1.50, (E) 3 PD50 booster 1.50–1.80, (F) 3 PD50 booster > 1.80. Arrows indicate the time of vaccination. Serum samples were collected before vaccination as day 0 and additional blood samples were obtained on days 28, 60, 90, and 120 postvaccination and analyzed via virus neutralization test. Horizontal red lines indicate the cut-off level of antibody titers for estimated protection. |
 | Fig. 3Arithmetic means of serum virus neutralizing antibody titers of calves vaccinated with 6 PD50 vaccine. (A) 6 PD50 single < 1.50, (B) 6 PD50 single 1.50–1.80, (C) 6 PD50 single > 1.80, (D) 6 PD50 booster < 1.50, (E) 6 PD50 booster 1.50–1.80, (F) 6 PD50 booster > 1.80. Arrows indicate the time of vaccination. Serum samples were collected before vaccination as day 0 and additional blood samples were obtained on days 28, 60, 90, and 120 postvaccination and analyzed via virus neutralization test. Horizontal red lines indicate the cut-off level of antibody titers for estimated protection. |
Table 1
Study groups according to vaccine potency, booster dose, and initial antibody level
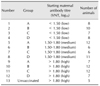
VNT, virus neutralization test. Group A, 3 PD50 single dose; Group B, 3 PD50 with booster; Group C, 6 PD50 single dose; Group D, 6 PD50 with booster. Experimental groups of vaccinated and unvaccinated calves were initially established according to the potency of the vaccine (3 PD50 or 6 PD50) and booster administration (A, B, C, and D). Later, the A–D groups were divided according to antibody titer level (low, medium, and high).
Acknowledgments
The authors thank Fuat Özyörük for useful advice and Banu Bayri Özbilge, Müslüm Kaan Arıcı, and Ayşenur Ulusoy for their excellent technical help. This work was supported by The Republic of Turkey, Ministry of Food, Agriculture and Livestock, General Directorate of Agricultural Research and Policy (grant No. TAGEM/HSGYAD/15/A02/P02/54).
References
1. Bielefeldt-Ohmann H, Prow NA, Wang W, Tan CS, Coyle M, Douma A, Hobson-Peters J, Kidd L, Hall RA, Petrovsky N. Safety and immunogenicity of a delta inulin-adjuvanted inactivated Japanese encephalitis virus vaccine in pregnant mares and foals. Vet Res. 2014; 45:130.

2. Bucafusco D, Di Giacomo S, Pega J, Juncos MS, Schammas JM, Pérez-Filgueira M, Capozzo AV. Influence of antibodies transferred by colostrum in the immune responses of calves to current foot-and-mouth disease vaccines. Vaccine. 2014; 32:6576–6582.

3. Chase CC, Hurley DJ, Reber AJ. Neonatal immune development in the calf and its impact on vaccine response. Vet Clin North Am Food Anim Pract. 2008; 24:87–104.

4. Council of Europe, European Pharmacopoeia Commission, European Directorate for the Quality of Medicines & Healthcare. European Pharmacopoeia. 8th ed. Strasbourg: Council of Europe, European Directorate for the Quality of Medicines and Healthcare;2013.
5. Davis EG, Bello NM, Bryan AJ, Hankins K, Wilkerson M. Characterisation of immune responses in healthy foals when a multivalent vaccine protocol was initiated at age 90 or 180 days. Equine Vet J. 2015; 47:667–674.

6. Dekker A, Eblé P, Stockhofe N, Chénard G. Intratypic heterologous vaccination of calves can induce an antibody response in presence of maternal antibodies against foot-and-mouth disease virus. BMC Vet Res. 2014; 10:127.

7. Downey ED, Tait RG Jr, Mayes MS, Garrick DJ, Ridpath J, Reecy JM. Effects of calf age and dam age on circulating BVDV II antibody levels prior to vaccination in Angus weanling calves. Reports No. 214. Ames: Iowa State Research Farm Progress Reports (US);2011.
8. Ellis J, Gow S, Bolton M, Burdett W, Nordstrom S. Inhibition of priming for bovine respiratory syncytial virus-specific protective immune responses following parenteral vaccination of passively immune calves. Can Vet J. 2014; 55:1180–1185.
9. Elnekave E, Zamir L, Hamd F, Even Tov B, Klement E. Risk factors for foot and mouth disease outbreaks in grazing beef cattle herds. Prev Vet Med. 2015; 120:236–240.

10. Endsley JJ, Roth JA, Ridpath J, Neill J. Maternal antibody blocks humoral but not T cell responses to BVDV. Biologicals. 2003; 31:123–125.

11. Foote MR, Nonnecke BJ, Beitz DC, Waters WR. Antigen-specific B-cell responses by neonatal calves after early vaccination. J Dairy Sci. 2007; 90:5208–5217.

12. Guzman E, Taylor G, Charleston B, Ellis SA. Induction of a cross-reactive CD8+ T cell response following foot-and-mouth disease virus vaccination. J Virol. 2010; 84:12375–12384.

13. Hodgins DC, Shewen PE, Dewey CE. Influence of age and maternal antibodies on antibody responses of neonatal piglets vaccinated against Mycoplasma hyopneumoniae. J Swine Health Prod. 2004; 12:10–16.
14. Jamal SM, Bouma A, van den Broek J, Stegeman A, Chénard G, Dekker A. Foot-and-mouth disease vaccine potency testing: the influence of serotype, type of adjuvant, valency, fractionation method, and virus culture on the dose-response curve in cattle. Vaccine. 2008; 26:6317–6321.

15. Knight-Jones TJ, Bulut AN, Gubbins S, Stärk KD, Pfeiffer DU, Sumption KJ, Paton DJ. Retrospective evaluation of foot-and-mouth disease vaccine effectiveness in Turkey. Vaccine. 2014; 32:1848–1855.

16. Knight-Jones TJ, Gubbins S, Bulut AN, Stärk KD, Pfeiffer DU, Sumption KJ, Paton DJ. Mass vaccination, immunity and coverage: modelling population protection against foot-and-mouth disease in Turkish cattle. Sci Rep. 2016; 6:22121.

17. Lee HS, Lee NH, Seo MG, Ko YJ, Kim B, Lee JB, Kim JS, Park S, Shin YK. Serological responses after vaccination of growing pigs with foot-and-mouth disease trivalent (type O, A and Asia1) vaccine. Vet Microbiol. 2013; 164:239–245.

18. Murphy JM, Hagey JV, Chigerwe M. Comparison of serum immunoglobulin G half-life in dairy calves fed colostrum, colostrum replacer or administered with intravenous bovine plasma. Vet Immunol Immunopathol. 2014; 158:233–237.

19. Nicholls MJ, Black L, Rweyemamu MM, Genovese J, Ferrari R, Hammant CA, de Silva E, Umehara O. The effect of maternally derived antibodies on the response of calves to vaccination against foot and mouth disease. J Hyg (Lond). 1984; 92:105–116.

20. Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol. 2014; 5:446.

21. Patil PK, Sajjanar CM, Natarajan C, Bayry J. Neutralizing antibody responses to foot-and-mouth disease quadrivalent (type O, A, C and Asia 1) vaccines in growing calves with pre-existing maternal antibodies. Vet Microbiol. 2014; 169:233–235.

22. Polewicz M, Gracia A, Buchanan R, Strom S, Halperin SA, Potter AA, Babiuk LA, Gerdts V. Influence of maternal antibodies on active pertussis toxoid immunization of neonatal mice and piglets. Vaccine. 2011; 29:7718–7726.

23. Sadir AM, Schudel AA, Laporte O, Braun M, Margni RA. Response to foot-and-mouth disease vaccines in newborn calves. Influence of age, colostral antibodies and adjuvants. Epidemiol Infect. 1988; 100:135–144.

24. Shankar H, Uppal PK. Immune response of newborn calves to vaccination with foot-and-mouth disease vaccine. Rev Sci Tech Off Int Epiz. 1982; 1:403–414.

25. Späth EJ, Smitsaart E, Casaro AP, Fondevila N, Fernández F, Leunda MR, Compaired D, Buffarini M, Pessi H. Immune response of calves to foot-and-mouth disease virus vaccine emulsified with oil adjuvant. Strategies of vaccination. Vaccine. 1995; 13:909–914.

26. Vidor E. Vaccination of newborns against hepatitis A in the presence of maternally derived antibodies. J Comp Pathol. 2007; 137:Suppl 1. S42–S45.

27. World Organisation for Animal Health (OIE). Foot and mouth disease. OIE. Terrestrial Animal Health Code. 20th ed. Paris: OIE;2011. p. 437–463.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download