Introduction
Lymphocytic plasmacytic enteritis (LPE), a chronic enteropathie, is the most frequently described type of inflammatory bowel disease in dogs [10,11,29]. LPE is recognized as one of the most common causes of chronic vomiting and diarrhoea [10,29]. The name of this disorder refers to the population of inflammatory cells present in the lamina propria of the small bowel.
Despite being an important disease, the exact aetiology remains unclear but appears to involve an exaggerated reaction of the mucosal immune system against the environment (bacteria and food antigens) in a susceptible host [12]. Some investigators have recently focused on the effect disturbing the homeostasis between the immune system and luminal antigens in the intestinal microenvironment [19]. Similarly, several reports have evaluated the role of cytokines [6,8,16,23,25], subpopulations of leukocytes [18,26], lymphocyte apoptosis [4], toll-like receptors [2], nuclear factor kappa-beta [19] or intestinal microbial communities [34].
Diagnosing inflammatory bowel disease is based on ruling out diseases that may cause intestinal inflammation along with histological evidence of inflammatory infiltration into the intestinal mucosa [10,11]. Marked advances in endoscopic equipment combined with the advantages of flexible endoscopy make upper gastrointestinal endoscopy the procedure of choice for obtaining intestinal biopsies to diagnose enteropathies in dogs and cats. Information about macroscopic parameters of gastric and duodenal mucosa evaluated during endoscopy can vary depending on who performs the technique. Recently, the gastrointestinal standardization group sponsored by World Small Animal Veterinary Association (WSAVA) proposed a set of guidelines for endoscopic examinations in order to obtain information about gastrointestinal endoscopic findings that could be universally used [31]. Moreover, the following should also be evaluated in the duodenum: distensibility of the lumen, hyperemia/vascularity, edema, discoloration, friability, texture, haemorrhage, erosion/ulcer, lacteal dilation, and contents (mucus, bile, or food).
Distended lacteals, defined as many expanded white villi in the duodenum, is an endoscopic finding strongly indicative of intestinal lymphangiectasia (IL) [20,24,28]. These scattered white spots have been described as having a snowflake-like or rice grain-like appearance [9]. Information about the presence of these white spots and their significance is limited. As far as we know, this finding has been reported in one dog with IL and LPE [17] and in eight dogs with ultrasonographic intestinal hyperechoic mucosal striations [27]. The aim of this retrospective study was to evaluate the significance of white spots in the duodenal mucosa of dogs with LPE.
Materials and Methods
We analysed endoscopy examination data that were compiled between January 2000 and December 2008 at the Complutense University of Madrid Veterinary Medical Teaching Hospital (Spain). All the dogs were cared for according to protocols approved by the Animal Experimentation Committee of the Complutense University of Madrid (Spain). The dogs in our study were diagnosed with LPE with white spots in the mucosa of the duodenum found by endoscopy (n = 22). Control dogs consisted of animals randomly selected during the review of the endoscopic data that had LPE but no white spots (n = 28). The following information was obtained from the medical records of all dogs: signalment, clinical signs, and physical examination findings and laboratory results at the time of presentation.
The severity of the clinical signs was assessed using two previously described activity indexes: the canine inflammatory bowel disease activity index (CIBDAI) [15] and canine chronic enteropathy clinical activity index (CCECAI) [1]. The CIBDAI is the sum of the score of six different clinical signs including attitude/activity, appetite, vomiting, stool consistency, stool frequency, and weight loss [15]. The recently introduced CCECAI, which is based on the previously established CIBDAI, also includes the scoring of serum-albumin concentration, peripheral edema and ascites, and severity of pruritus [15]. The minimum information obtained for each dog included a complete anamnesis, complete blood count and serum biochemical profile; fecal examination for three consecutive days for cestodes, nematodes, and protozoa (direct smear with saline solution, direct smear with methiolate iodine formaldehyde solution and zinc-sulfate flotation, or the Telemann technique); fecal chymotrypsin, and serum trypsin-like immunoreactivity. Persistent gastrointestinal signs (> 3 weeks in duration) in combination with normal results from a thorough diagnostic evaluation and absence of a response to diet modification (prescription dry diets for gastrointestinal disease with low concentration of lipid) were noted in these dogs. Furthermore, the lack of a good response to antibiotic therapy was also noted.
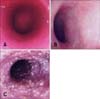
Gastroduodenoscopy was performed in all dogs in order to obtain gastric and duodenal mucosal biopsy specimens. Six to eight samples were taken from each area, as previously recommended [32]. Videoendoscopes of variable lengths and diameters were used according to the size of the dog for endoscopic exploration. Dogs were denied access to food 24 h prior to endoscopy and to water 12 h before the examination. An average of five to six images showing representative findings (through the descending duodenum) were routinely captured by a video printer. For all animals, photographs of the duodenum with gross white spots were reviewed in order to assess the density of the spots (1 = mild, 2 = moderate, 3 = severe). This evaluation method was chosen by the authors after taking into account the scores for other endoscopic parameters proposed by the WSAVA gastrointestinal standardization group [31]. The density of the white spots was graded by one of the authors (FR). Information regarding the animals' medical history, clinical signs, laboratory results, or histopathologic descriptions was not available to the clinician when evaluating the spots.
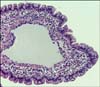
All duodenal mucosal biopsy specimens were taken using flexible, through-the-endoscope, pinch biopsy forceps with smooth-edged oval cups. Biopsy specimens were fixed by immersion in neutral-buffered 10% formalin, embedded in paraffin wax, cut into 5 µm-thick sections, and stained with hematoxylin and eosin, Masson trichromic, and periodic acid-Schiff reagent. LPE was diagnosed in each dog based on information from the diagnostic evaluation combined with a histopathologic finding of lymphocyte and plasma cell infiltration into the lamina propria of the duodenum [7]. A complete histopathological evaluation of all biopsies was performed according to the histopathologic criteria recently proposed by the WSAVA gastrointestinal standardization group for diagnosing gastrointestinal inflammation in dogs and cats [5]. Specifically, the findings for cases of lacteal dilation were scored from 0 to 3 as follows: 0 = normal, when the central lacteal represented up to 25% of the villous lamina propria width on the longitudinal section; 1 = mild dilation, when this width represents up to approximately 50%; 2 = moderate dilation, when this width represented up to 75%; and 3 = marked dilation, when central lacteal represented up to 100% of the villous lamina propria [5].
Statistical analysis was performed using commercially available software (IBM SPSS Statistics 19; SPSS, USA). The clinical and histological findings of the two LPE groups (with and without white spots) were compared. Data were analysed using Student's t-tests or Willcoxon signed-rank tests depending on the distribution of the variables. Correlations between the density of the white spots in the duodenum, serum proteins, serum albumin, activity index, and histological grading were analysed in LPE group with white spots using Spearman's test. A chi-square test was used to compare percentages; a Fisher exact test was used when needed. A p value of less than 0.05 was considered statistically significant.
Results
The LPE group with white spots in the duodenum included 22 animals: 17 males and 5 females. The median age at the time of endoscopy was 5.2 years (range, 2~10 years). Six animals were mixed breeds along with 16 dogs that were 11 different pure breeds. The LPE group without white spots in the duodenum included 28 animals with 18 males and 10 females. The median age at the time of endoscopy was 5 years (range, 2~9 years). Seven dogs were mixed breeds and 21 dogs were 12 different pure breeds. Statistical significant differences were not found between both groups regarding age, sex and breed distribution.
Decreased serum protein concentrations (≤ 5.6 g/dL) were found in 31.8% (7/22) of the LPE dogs with white spots and in 7.1% (2/28) of the LPE dogs without white spots. Hypoproteinemia was significantly more frequent in dogs with white spots compared to ones without (p = 0.02). Mean serum protein concentrations were 5.68 g/dL (range, 2.4~8.4 g/dL) in LPE dogs with white spots and 6.58 g/dL (range, 4.6~7.6 g/dL) in LPE dogs without. Serum protein concentration was significantly lower in white spots LPE dogs related to the other group (p = 0.038). Mean serum albumin concentrations were 2.84 g/dL (range, 1.0~4.3 g/dL) in LPE dogs with white spots and 3.29 g/dL (range, 2.3~4 g/dL) in ones without. Serum albumin concentrations were significantly lower in the LPE dogs with spots compared to the dogs without (p = 0.039).
No significant differences in activity indices were observed between the two groups according to CCECAI (white spots group: mean, 4.54; range, 0~15; group without spots: mean 5.53; range, 2~14). CIBDAI scores were significantly lower (p = 0.016) in the white spots group (mean, 3.77; range, 0~11) compared to the group without white spots (mean, 5.21; range, 2~9). Lacteal dilation histological scores were significantly higher (p = 0.027) in LPE dogs with white spots (mean, 0.45; range, 0~2) compared to dogs without white spots (mean, 0.12; range, 0~1). No statistically significant differences were found between the two groups when analyzing the other histological parameters.
The density of the white spots in the duodenal mucosa was graded as mild in 14 dogs (64%; Fig. 1A), moderate in four (18%; Fig. 1B), and severe in four dogs (18%; Fig. 1C). Hypoproteinemia was found in 21% of the dogs (3/14) with mild white spot density, in 25% (1/4) with moderate density, and in 75% (3/4) with severe density. No statistically significant correlation was found between density of white spots and serum protein or albumin concentrations. Likewise, no statistically significant correlation was found between the density of the white spots and either disease activity indices (CIBDAI, p = 0.894; CCECAI, p = 0.076). However, the results showed a statistically significant correlation between density of the white spots and lymphatic dilatation histological scores (p = 0.023; ρ = 0.481; Fig. 2).
Discussion
To date, the present study is the first that compares two populations of LPE dogs, with or without white spots in the duodenal mucosa. The characteristics of both populations were similar in terms of age, gender, and breed distribution. Until now, the appearance of many white spots on endoscopy strongly suggested intestinal IL [9,20,24,28,30]. To our knowledge, this feature has not been routinely described in dogs with LPE.
According to our results, the presence of hypoproteinemia in LPE dogs is significantly higher when white spots are present in the duodenal mucosa than when they are not. Previous studies reported a higher prevalence of hypoproteniemia in these LPE dogs compared to our study, ranging from 24~63% [3,13,14,21]. It is unclear why the LPE dogs in the present study had a lower prevalence of hypoproteinemia compared to previous studies. These results are in accordance with previous experience of the group with this disease. Geographical differences cannot be excluded.
Although not statistically significant, correlation between serum protein or albumin levels and the density of the white spots was found. Significant lower serum protein and albumin concentrations in LPE dogs with white spots was probably related to the presence of these spots in the duodenal mucosa. A recent study was the first to describe a significant association between lacteal dilation and hypoalbuminemia [33]. An increase in plasma proteins leakage from the intestine can be the result of several causes via one of two main mechanisms: 1) mucosal injury with or without erosions or ulcerations, and 2) increased lymphatic pressure in the gut due to different factors. In our dogs, both mechanisms could be implicated. Rupture of intestinal villous lacteals that appeared as "rice-grain" spots in duodenum in our study indicated lymph leakage into the intestinal lumen with other components such as chylomicrons, lymphocytes, and proteins [20,22]. Although some lymph constituents can be digested and reabsorbed at more distal sites in the intestine, the presence of lacteals dilation, inflammation, or oedema in the mucosa can limit intestinal absorptive capacity, resulting in a net loss of lymph [20].
It was surprising that CIBDAI scores was significantly lower in the group with white spots compared to the group without. In this study, LPE dogs with white spots had significant lower serum albumin concentrations. Hypoalbuminemia has been reported to be a sign of poor prognosis [1,3] which is why this group of dogs was expected to be in a poor clinical condition. After reviewing the activity indices of this group, we hypothesised that a possible reason for the relatively low CIBDAI scores might be that these animals presented only one clinical sign like vomiting or severe abdominal pain that is not noted in this activity index.
Up to now, the presence of scattered white spots in the duodenal mucosa seems to correspond to lacteals that are dilated and filled with chyle [9,24]. The results from our study support this hypothesis based on the significant correlation found between the histological grade of lacteals distension and density of the white spots observed during endoscopy exploration. In cases of IL (congenital or acquired), the intestinal villous lacteals can dilate, become more fragile, and rupture easily when pressure in the mesenteric or intestinal lymph vessels increases independently of the disease [20]. This explanation could also explain the presence of white spots in cases of LPE because distended lacteals are frequently found in the presence of this disease [10]. In animals with intestinal diseases such as inflammatory LPE, processes are suspected of blocking lymph flow [20].
The results obtained from this study suggest that the appearance of white spots in the duodenal mucosa of dogs is not a finding exclusive to IL. Low serum protein and albumin concentrations are probably a result of these white spots. In our study, the density of the white spots in the duodenum significantly correlated with the histological lacteal dilation scores. Further studies are needed in order to evaluate the clinical significance of these white spots in dogs with LPE, especially in terms of prognosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download