Abstract
A malignant myoepithelioma is one of the rarest salivary gland neoplasms which may either arise de novo or develop within a preexisting pleomorphic adenoma or benign myoepithelioma. The parotid gland is the most common primary site and the palate the most common intra-oral site of occurrence. Herein is present a case of a malignant myoepithelioma arising in the hard palate of a 79-year-old woman. The lesion had been examined by biopsy at another hospital, and diagnosed as a poorly differentiated squamous cell carcinoma. The patient underwent a wide local tumor resection. Examination of the resection specimen showed the characteristic histopathological and immunohistochemical features of a malignant myoepithelioma. Five months after the operation, the patient was well without evidence of recurrence or metastasis.
Salivary gland tumors displaying exclusively myoepithelial differentiation are referred to as myoepitheliomas. Myoepitheliomas are rare tumors that account for less than 1% of all salivary gland tumors.1,2 There seems to be a range of differentiation among the myoepitheliomas, with both benign and malignant variants represented. The majority of myoepitheliomas reported in the literature have been benign, and approximately 50 malignant myoepithelioma cases have been reported in the English literature, mostly as single case reports.3 The rarity contrasts with the active role of myoepithelial cells in the histogenesis of several types of salivary gland tumors.4 It seems that malignant myoepitheliomas have been underrecognized in the past and are probably not as rare as previously thought.3 A malignant myoepithelioma may arise de novo or develop within a preexisting pleomorphic adenoma or benign myoepithelioma.1,5,6 Similarly to benign myoepitheliomas, the parotid gland is the most common primary site and the palate the most common intra-oral site of occurrence.3,7
In this report, a case of a malignant myoepithelioma arising in a 79-year-old woman is presented, and the clinicopathological and immunohistochemical aspects of such tumors is discussed.
A 79-year-old woman presented with a 4-month history of a painless swelling in the hard palate. The swelling had gradually increased in size over the last two months. The lesion had been examined by biopsy at another hospital, and diagnosed as a poorly differentiated squamous cell carcinoma. The biopsy specimen was reviewed at our hospital, and the tumor diagnosed as a poorly differentiated carcinoma.
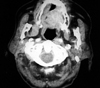
Oral examination revealed a 3×4 cm firm, painless, submucosal mass located in the hard palate and overlying mucosa was ulcerated. There was no regional lymphadenopathy. The remainder of her physical examination was otherwise normal and laboratory studies showed no abnormalities. Magnetic resonance imaging of the head and neck region revealed a solid mass in the hard palate (Fig. 1). The tumor was removed using a transoral approach under general anesthesia. The lesion was mapped with an adequate margin of 1 cm and the incision included the periosteum. No invasion was seen on the periosteum and bone. The defect was covered with a dermal graft.
The postoperative course of the patient was uncomplicated, and she was discharged on the 12th postoperative day. Five months after the operation, the patient is well without evidence of recurrence or metastasis.
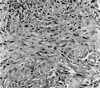
Macroscopically, the tumor was 3×4 cm in size and unencapsulated. The overlying mucosa was ulcerated. The cut surface of the tumor was solid and tan-white, with punctate yellowish foci. Microscopically, the tumor was composed of a mixture of two distinct neoplastic cell populations. The predominant population was composed of large polyhedral cells with eosinophilic cytoplasm and eccentric, round nuclei (Fig. 2). The cells had a distinct plasmacytoid appearance. The nuclei were generally large, hyperchromatic and pleomorphic, and contained prominent nucleoli. The cells were arranged in either sheets or as loose aggregates lying in a myxoid matrix. The mitotic rate was 18 mitoses per 10 high power fields (HPFs) and atypical mitotic figures were also observed. The second population was seen in focal areas and consisted of spindle, fibroblast-like cells with elongated nuclei. The cells formed bundles and showed less pleomorphism than the plasmacytoid cells. There were small foci of necrosis. No perineural and vascular invasions were observed. Noncohesive, infiltrative single tumor cells were seen at the edge of the tumor (Fig. 3). The surgical margins were free of disease.
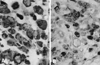
The tumor cells were strongly and diffusely positive for vimentin (Neomarkers, Fremont, CA, USA) and S-100 protein (Novocastra, Newcastle, UK) (Fig. 4). Large numbers of tumor cells were immunoreactive for cytokeratin (Neomarkers). Epithelial membrane antigen (Neomarkers), smooth muscle actin (Novocastra) and glial fibrillary acidic protein (Dako, Carpinteria, CA, USA) were also expressed focally. The tumor cells were negative for desmin (Novocastra) and HMB-45 (Neomarkers). The Ki-67 (using the mAb MIB-1, Novocastra) labeling index was 17%.
Malignant myoepithelioma is one of the rarest salivary gland neoplasms. It was first described by Stromeyer et al.8 in 1975 and approximately 50 cases have subsequently been reported in the English literature.3 Malignant myoepithelioma was included in the updated histological classification of salivary gland tumors by the World Health Organization in 1991.9
Patients with malignant myoepithelioma are generally aged over 50 years1,3 and the majority presents with a painless mass as the primary complaint.3 The parotid gland is the most common primary site,1,3 followed by the submandibular gland and minor salivary glands.10,11 The palate is the most common intraoral site of occurrence.3,7
Grossly, these tumors are generally soft to slightly firm and unencapsulated. They have infiltrative tumor borders with destructive tumor extensions into the adjacent salivary gland or surrounding tissues.3 The tumor cells in malignant myoepithelioma patients show a wide variety of morphology, comprising of spindle, plasmacytoid (hyaline), epithelioid and clear cell subtypes and combinations of these cell types may be present within the same tumor. In malignant myoepitheliomas, two different tumor-related matrices have been described: myxoid and hyalinized. In some malignant myoepithelioma cases metaplastic changes have been noted,1,3 including squamous, chondroid and sebaceous metaplasia. Immunohistochemically, the combined expressions of myoepithelial markers, such as vimentin, S-100 protein, glial fibrillary acidic protein and smooth muscle actin, in addition to cytokeratins, are characteristic.1,12
To establish the diagnosis of a malignant myoepithelioma, two histologic criteria must be satisfied: the neoplastic cells must show exclusively myoepithelial differentiation and the tumor must exhibit malignant features.5,10,12 In our case, the immunoreactivities of the tumor cells for S-100 protein, cytokeratins, vimentin, glial fibrillary acidic protein (GFAP), and smooth muscle actin were in agreement with a myoepithelial phenotype. Additionally, the lack of ductal and acinar differentiation also supported the diagnosis of a myoepithelial tumor. Increased mitotic activity, cellular pleomorphism and necrotic areas, and more importantly, an infiltrative and destructive growth pattern favored the diagnosis of malignancy.
The differential diagnosis of a malignant myoepithelioma depends on the predominant cell type. Plasmacytoid cell type malignant myoepitheliomas should be distinguish from a plasmacytoma, malignant melanoma and large cell lymphoma. For the spindle cell type, the differential diagnosis includes hemangiopericytoma, schwannoma, fibrosarcoma, leiomyosarcoma and malignant peripheral nerve sheath tumor. Immunohistochemical staining is helpful in differentiating these lesions. A malignant myoepithelioma should also be distinguish from its benign counterpart. The histological features considered helpful in discriminating benign and malignant myoepitheliomas include cytological atypia, mitotic activity, infiltrative growth pattern and necrosis.1,11,13,14 Savera, et al.3 emphasized that the minimum requirement for the diagnosis of a malignant myoepithelioma is the presence of tumor infiltration into the adjacent tissues. Recently, it has been shown that more than seven mitoses per 10 HPFs or a Ki-67 labeling index of more than 10% is diagnostic of malignant myoepithelioma.1 In the oral region, distinction between a malignant myoepithelioma and spindle cell or poorly differentiated squamous cell carcinoma can be challenging, especially with small biopsy specimens. In our case, the lesion had been examined by biopsy at another hospital, and diagnosed as a poorly differentiated squamous cell carcinoma. However, examination of the resection specimen showed characteristic histopathological and immunohistochemical features of a malignant myoepithelioma.
The clinical and biological behavior of these tumors is variable. There are no definite histological features that correlate clearly with their behavior. The influence of various parameters (tumor size, site, cell type, cytologic grade, presence of underlying benign tumor, mitotic rate, necrosis, perineural and vascular invasion) on the prognosis was studied by Savera, et al.,3 and they found cytologic atypia correlated weakly with a poor outcome, but none of the other factors showed a significant correlation. Similarly, Nagao, et al.1 observed no apparent association between the cell types and clinical behavior of malignant myoepitheliomas. The prognostic implication of the histogenesis of malignant myoepitheliomas is controversial. Nagao, et al.1 found no differences in the outcome with regard to the presence or absence of a pre-existing pleomorphic adenoma, while Di Palma and Guzzo13 considered a malignant myoepithelioma as a low grade malignancy, characterized by multiple recurrences and a long clinical history when arising from a pleomorphic adenoma, but tend to be more aggressive and have a short clinical history when arising de novo. Nagao, et al.1 showed that high proliferative activity, extensive invasion into the surrounding tissues, perineural permeation and marked cellular pleomorphism were correlated with a poor prognosis.
There is little information about the treatment of these tumors to date, however, wide surgical excision is accepted as the appropriate treatment modality. Therapeutic neck dissection is indicated when there are clinically or radiologically apparent metastases in the cervical lymph nodes.4 Although the value of radiotherapy is unknown, it may be useful in patients with higher-stage and higher-grade neoplasms.15
Figures and Tables
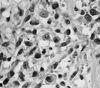 | Fig. 2Plasmacytoid and spindle-shaped cells, with pleomorphic nuclei and prominent nucleoli in a myxomatous stroma (H&E, original magnification×200). |
References
1. Nagao T, Sugano I, Ishida Y, Tajima Y, Matsuzaki O, Konno A, et al. Salivary gland malignant myoepithelioma: a clinicopathologic and immunohistochemical study of ten cases. Cancer. 1998. 83:1292–1299.
2. Sciubba JJ, Brannon RB. Myoepithelioma of salivary gland: report of 23 cases. Cancer. 1982. 49:562–572.

3. Savera AT, Sloman A, Huvos AG, Klimstra DS. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000. 24:761–774.
4. Dean A, Sierra R, Alamillos FJ, Lopez-Beltran A, Morillo A, Arévalo R, et al. Malignant myoepithelioma of the salivary glands: clinicopathological and immunohistochemical features. Br J Oral Maxillofac Surg. 1999. 37:64–66.

5. McCluggage WG, Primrose WJ, Toner PG. Myoepithelial carcinoma (malignant myoepithelioma) of the parotid gland arising in a pleomorphic adenoma. J Clin Pathol. 1998. 51:552–556.

6. Tralongo V, Rodolico V, Burruano F, Tortorici S, Mancuso A, Daniele E. Malignant myoepithelioma of the minor salivary glands arising in a pleomorphic adenoma. Anticancer Res. 1997. 17:2671–2675.
7. Guzzo M, Cantù G, Di Palma S. Malignant myoepithelioma of the palate: report of case. J Oral Maxillofac Surg. 1994. 52:1080–1082.

8. Stromeyer FW, Haggitt RC, Nelson JF, Hardman JM. Myoepithelioma of minor salivary gland origin. Light and electron microscopical study. Arch Pathol. 1975. 99:242–245.
9. Seifert G, Sobin LH. Histological typing of salivary gland tumours. (WHO. World Health Organization. International Histological Classification of Tumours.). 1991. 2nd ed. New York: Springer-Verlag.
10. Suba Z, Németh Z, Gyulai-Gaál S, Ujpál M, Szende B, Szabó G. Malignant myoepithelioma. Clinicopathological and immunohistochemical characteristics. Int J Oral Maxillofac Surg. 2003. 32:339–341.

11. Tuncel U, Ergul G, Ozlugedik S, Unal A. Myoepithelial carcinoma in the nasopharynx: an unusual localization. Yonsei Med J. 2004. 45:161–165.

12. Ogawa I, Nishida T, Miyauchi M, Sato S, Takata T. Dedifferentiated malignant myoepithelioma of the parotid gland. Pathol Int. 2003. 53:704–709.

13. Di Palma S, Guzzo M. Malignant myoepithelioma of salivary glands: clinicopathological features of ten cases. Virchows Arch A Pathol Anat Histopathol. 1993. 423:389–396.

14. Lauro S, Trasatti L, Larosa G, Calabretta F, Di Gioia CR, Vecchione A. Myoepithelial carcinoma of parotid gland: a case report. Anticancer Res. 2003. 23:3041–3044.
15. Gnepp DR, El-Mofty SK. Damjanov I, Linder J, editors. Salivary glands. Anderson's Pathology. 1996. 10th ed. St. Louis: Mosby;1616–1646.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download