Abstract
Posterior reversible encephalopathy syndrome (PRES) is usually a reversible clinical and radiological entity associated with typical features on brain MR or CT imaging. However, the not-so-uncommon atypical radiological presentations of the condition are also present and they may go unrecognised as they are confused with other conditions. Here, we report a very rare case of atypical, unilateral PRES in a 49-year-old uremic, post-transplant female patient who presented with seizures. Initial MRI showed high-grade occlusion of the left middle cerebral artery (MCA) and lesions suggestive of subacute infarction in the ipsilateral frontotemporoparietal lobe. Patient symptoms had resolved a day after the onset without any specific treatment but early follow-up CT findings suggested hemorrhagic transformation. Follow-up MRI performed 2 years later showed complete disappearence of the lesions and persisting MCA occlusion.
Posterior reversible encephalopathy syndrome (PRES) is usually a reversible radiological and clinical entity which presents with a variety of symptoms ranging from headache, seizures, visual disturbances, altered mental status, and loss of consciousness. The most common causes of the syndrome are severe hypertension, eclampsia, bone marrow or solid organ transplantation, immunosuppressive drug toxicity, renal failure, infection, and various autoimmune diseases (12). Typical imaging manifestations are bilateral symmetric areas of hemispheric edema involving the parieto-occipital cortical-subcortical regions which usually do not enhance and show restricted diffusion. However, atypical imaging findings in terms of localization, asymmetry and unilaterality of the lesions, diffusion restriction, contrast enhancement, hemorrhage, and irreversibility have been described in the literature (3456) and they are not as uncommon as expected. To the best of our knowledge, there are only a few reported cases of unilateral involvement by PRES. Herein, we describe a rare atypical case of unilateral PRES mimicking a middle cerebral artery (MCA) infarction and it was diagnosed retrospectively two years later during follow-up. This report was approved by the Institutional Review Board (IRB file No. 4-2014-0786).
A 49-year-old cachectic female patient with a body weight of 30 kg who was hospitalized for prerenal acute renal failure due to poor oral intake developed tonic clonic seizures that lasted for two minutes and showed signs of confusion afterwards. On laboratory analysis, she had an increased blood urea nitrogen level of 30.5 mg/dL and a creatinine level of 1.09 mg/dL. Serum electrolyte levels, hepatic function tests, and bleeding parameters were within normal limits. Her complete blood count was normal except for anemia (hemoglobin: 11.3 g/dL). She did not have any signs of infection and had negative blood and urine cultures. She was slightly hypertensive with a maximum systolic blood pressure of 140 mm Hg and a diastolic blood pressure of 90 mm Hg. She did not have any known history of a neurological disease but had undergone bilateral lung transplantation due to idiopathic pulmonary fibrosis 6 months ago and was on immunosuppressive treatment with tacrolimus. Plasma tacrolimus concentration was 8 ng/mL and it was within normal limits. She underwent magnetic resonance imaging (MRI) examination of the brain. On MRI, there were extensive hyperintense lesions predominantly in the subcortical and deep white matter of the left frontotemporoparietal lobe including the splenium of the corpus callosum and basal ganglia on fluid attenuation inversion recovery (FLAIR) images (Fig. 1A). The lesions were iso to hyperintense on diffusion-weighted images (DWI) (Fig. 1B) and hyperintense on apparent diffusion coefficient (ADC) maps without any signs of restricted diffusion (Fig. 1C). On contrast-enhanced three-dimensional T1-weighted images, lesions showed patchy enhancement (Fig. 1D). Time-of-flight MR angiogram revealed high-grade occlusion of the left MCA (Fig. 1E), but she did not undergo any further imaging with computed tomography (CT) or conventional angiography. The imaging findings were interpreted as a subacute infarction in the left MCA territory and the patient was given aspirin therapy. The patient did not experience any other seizures and her mental confusion disappeared the next day. She underwent follow-up CT imaging 5 days later and it showed a newly developed small intraparenchymal hematoma in the left temporal lobe which was interpreted as hemorrhagic transformation. Right frontal subdural widening and a small subarachnoid hemorrhage along the right side of the anterior interhemispheric fissure were also noted (Fig. 1F). Aspirin was stopped immediately. The patient had a short-term follow-up with CT in the first two weeks and did not have a follow-up MRI examination until two years later. MRI examination performed two years later revealed complete disappearence of the lesions along with persisting left MCA occlusion and an old hemorrhagic focus in the left temporal lobe (Fig. 1G, H). This reversibility of ischemic lesions was quite unexpected and led to re-evaluation of the first MRI examination of the patient. ADC hyperintensity which is representative of vasogenic edema, contrast enhancement, involvement of the splenium of the corpus callosum which is supplied by posterior circulation rather than the MCA, initially neglected presence of subtle hyperintensity in the contralateral external capsule (Fig. 1A), and parenchymal hemorrhage led to the retrospective diagnosis of atypical unilateral presentation of PRES in the appropriate clinical setting.
Posterior reversible encephalopathy syndrome was first described in 1996 in patients with radiological findings suggestive of white matter edema in the posterior parieto-temporo-occipital region (1). Since then, there have been many reports attempting to highlight the spectrum of causes, mechanisms, and imaging findings of this syndrome that made the term 'PRES' rather unsatisfactory as the lesions are not always confined to the white matter and posterior regions, and they are not always reversible.
The typical MRI findings of this syndrome are symmetric hyperintensity in bilateral parietooccipital cortical-subcortical white matter (98%) on FLAIR images. However, the frontal lobe (68%), temporal lobe (40%), cerebellum (30%), basal ganglia, brain stem, and deep white matter can also be involved. The involvement of brain stem or basal ganglia with sparing of the subcortical regions was named as "central-variant" PRES (4%) and involvement of the splenium of the corpus callosum was distinctly reported in 10% of the patients (345). On DWI, lesions are usually isointense or less commonly hyperintense due to T2 shine-through effect and hyperintense on ADC maps suggesting vasogenic edema. Restricted diffusion representing cytotoxic edema is also observed occasionally and it is associated with irreversible infarction (7). Lesions usually do not show contrast enhancement; however, leptomeningeal, gyriform, or cortical enhancement may be encountered (37.7%) (3).
Intracranial hemorrhage is known to occur in PRES and it could present as intraparenchymal hematoma, sulcal subarachnoid hemorrhage, and minute hemorrhages. The reported rate of hemorrhage was 5-17% on CT and conventional MRI (38). However, the incidence of micro-hemorrhages was much higher (60%) with susceptibility-weighted imaging (9).
The involvement in PRES could be asymmetrical or rarely unilateral. The main imaging differential diagnoses of PRES are ischemia, cerebral venous thrombosis, acute disseminated encephalomyelitis, Creutzfeldt-Jakob disease, progressive multifocal leukoencephalopathy, and neoplastic conditions (5).
Regarding the pathogenesis, autoregulatory failure and endothelial injury have been proposed. Autoregulation is the maintenance of a constant blood flow in the brain by arteriolar constriction and dilatation regulated by sympathetic innervation. Sudden elevation of systemic blood pressure could exceed the capacity of autoregulation and the arterioles dilate resulting in brain hyperperfusion, blood brain barrier breakdown, and extravasation of fluid and blood products. This could also explain the posterior predilection of the syndrome as the arteries of the posterior circulation have relatively poorer sympathetic innervation (1). However, the reported blood pressure usually does not exceed the autoregulatory capacity (mean arterial pressure > 150-160 mm Hg), extent of vasogenic edema does not correlate with the severity of hypertension, and PRES could also be encountered in normotensive patients (20-40%). Therefore, autoregulatory failure does not suffice for explanation. PRES is almost exclusively seen in the setting of a significant systemic process/condition, including transplantation, infection/sepsis/shock, eclampsia, autoimmune disease, and postcancer chemotherapy. The underlying mechanisms are similar in these conditions and are mediated by a cascade of events starting with immune system activation causing endothelial injury and resulting in vasogenic edema (2). Our patient had multiple risk factors for development of PRES in terms of uremia, prior lung transplantation, and tacrolimus usage. Although plasma tacrolimus levels were normal, toxic levels are not imperative; and even after several months of exposure to the drug, patients with therapeutic levels can be symptomatic (7). Our assumption is that unilateral involvement in our patient might have been the result of exposure to the combined effects of risk factors causing endothelial injury in a more vulnerable brain region due to chronic vascular occlusion. Although we cannot totally exclude the possibility, we do not think that hypertension was the central pathophysiologic phenomenon in our case as she was only mildly hypertensive and did not experience hypertension that was above her baseline or exceed the limits of autoregulation that coincided with her symptom onset.
There have also been a few other cases of unilateral or asymmetrical PRES reported in the literature. The common mechanism in most of these cases was proposed as autoregulatory failure and hyperperfusion caused by severe hypertension, and all cases had a vascular abnormality such as chronic occlusive vascular disease (10), subarachnoid hemorrhage-associated vasospasm (611), and a hyperplastic anterior choroidal artery (12). Unilateral or asymmetrical lesions were in the territory of the vascular abnormality in cases of chronic hypoperfusion and hyperplastic artery as in our case, whereas these territories were spared in cases of new-onset hypoperfusion like subarachnoid hemorrhage-associated vasospasm. In two other cases, unilateral involvement was associated with cyclosporine toxicity, albeit no information regarding vascular structures was available (3).
In conclusion, awareness of atypical findings of PRES is important as they are more common than perceived and for the prompt treatment of the condition because it is usually reversible after eliminating the offending cause. We propose that altered hemodynamics caused by an underlying vascular disease could be responsible for the unilateral or asymmetrical involvement.
Figures and Tables
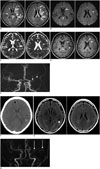 | Fig. 1Unilateral posterior reversible encephalopathy syndrome in 49-year-old woman with acute renal failure who had history of lung transplantation and of taking tacrolimus.
A. Axial fluid attenuation inversion recovery images show extensive hyperintense lesions mainly in left frontotemporoparietal region predominantly involving subcortical and deep white matter including splenium of corpus callosum, and striatum (short arrows). Subtle hyperintensity is also noted in right external capsule (long arrow). B. Diffusion weighted images show that lesions are iso to hyperintense (arrows). C. Apparent diffusion coefficient maps show that lesions are hyperintense suggesting vasogenic edema (arrows). D. Post-contrast axial three-dimensional (3D)-T1 weighted images show patchy enhancement of lesions (arrows). E. 3D-time-of-flight image reveals high-grade occlusion of left middle cerebral artery (arrows). F. Follow-up CT examination of patient performed 5 days later. Axial image shows newly developed small intraparenchymal hematoma in left temporal lobe (asterisk). Right frontal subdural widening and small subarachnoid hemorrhage along right side of anterior interhemispheric fissure were also noted (arrow). Follow-up MRI performed at two years after first examination. G. Axial fluid attenuation inversion recovery images show complete disappearance of lesions and old hemorrhagic focus in left temporal lobe (arrow). H. Three-dimensional time-of-flight image shows stable left middle cerebral artery occlusion (arrows).
|
References
1. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996; 334:494–500.
2. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008; 29:1036–1042.
3. McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007; 189:904–912.
4. McKinney AM, Jagadeesan BD, Truwit CL. Central-variant posterior reversible encephalopathy syndrome: brainstem or basal ganglia involvement lacking cortical or subcortical cerebral edema. AJR Am J Roentgenol. 2013; 201:631–638.
5. Hugonnet E, Da Ines D, Boby H, Claise B, Petitcolin V, Lannareix V, et al. Posterior reversible encephalopathy syndrome (PRES): features on CT and MR imaging. Diagn Interv Imaging. 2013; 94:45–52.
6. Schambra HM, Greer DM. Asymmetric reversible posterior leukoencephalopathy syndrome. Neurocrit Care. 2006; 4:245–247.
7. Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002; 23:1038–1048.
8. Hefzy HM, Bartynski WS, Boardman JF, Lacomis D. Hemorrhage in posterior reversible encephalopathy syndrome: imaging and clinical features. AJNR Am J Neuroradiol. 2009; 30:1371–1379.
9. McKinney AM, Sarikaya B, Gustafson C, Truwit CL. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012; 33:896–903.
10. Dhar R, Dacey R, Human T, Zipfel G. Unilateral posterior reversible encephalopathy syndrome with hypertensive therapy of contralateral vasospasm: case report. Neurosurgery. 2011; 69:E1176–E1181. E1181
11. Voetsch B, Tarlov N, Nguyen TN, DeFusco C, Barest GD, Norbash A, et al. Asymmetric posterior reversible encephalopathy syndrome complicating hemodynamic augmentation for subarachnoid hemorrhage-associated cerebral vasospasm. Neurocrit Care. 2011; 15:542–546.
12. Romano A, Silvia P, Alberto P, Tavanti F, Sette G, La Starza S, et al. Asymmetric posterior reversible encephalopathy syndrome in patient with hyperplastic anterior choroidal artery. J Headache Pain. 2011; 12:259–261.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download