INTRODUCTION
Heart failure (HF) is one of major causes of death in developed countries, and ischemic heart disease, e.g., myocardial infarction (MI), is an important contributor to HF.
1)2) Prolonged myocardial ischemia causes cardiomyocyte (CMC) necrosis and myocardial fibrosis.
3) So, replacement of cells in the ischemic region can be an important therapeutic modality for medically intractable ischemic HF.
3) The ultimate purpose of cell therapy is to repair cardiac function by replacing damaged CMCs.
3) Resident cardiac progenitor cells (CPCs) can be used as an candidate source of stem cell therapy for ischemic HF, because CPCs can proliferate, differentiate, be mobile, and form tubes to make new vessels in either in vivo or in vitro.
3) CPC was firstly reported in 2003 as being able to self-renew and proliferate.
4) Also, CPCs can differentiate into certain cell types, such as CMC, endothelial cells (ECs), and vascular smooth muscle cells (VSMCs) in vivo.
4) Consequently, CPCs have the potential to repair damaged myocardial tissue provoked by acute MI or chronic myocardial ischemia.
3) Interestingly, previous studies reported that CPCs injected into myocardial tissue could differentiate into CMCs and ECs, thus improving myocardial systolic function.
5) Basically, CPCs injected into ischemic jeopardized myocardium can migrate to the ischemic area and form new vessels to supply oxygen, nutrients, and various cytokines to promote healing of myocardium.
5) So, the ability of CPCs to migrate and form tubes can be important characteristics to rescue ischemic myocardium.
The arrestin family consists of arrestin 1 to 4, which mainly reside in the retinal rods and cones, and arrestin 2, 3 (newly named β-arrestin 1, 2; β-arrs), which are ubiquitous in mammalian cells.
6) β-arrestin1 (β-arr1) and β-arrestin2 (β-arr2) act as adaptor and scaffold proteins of 7 transmembrane domain receptors, or G-protein coupled receptors (GPCRs), and lead to desensitization and internalization of GPCRs.
6) Aside from this canonical action of β-arrs on GPCR, these proteins play important roles in regulating cell proliferation, migration, and tube formation related to various signaling pathways in certain cell types.
6) In addition, β-arrs regulate cytoskeleton and actin reorganization, and affect migration of ECs or malignant cells.
7) β-arrs action for promoting cell migration requires extracellular signal-regulated kinase (ERK) pathway activation within the leading edge of migrating cells.
7) β-arrs dependent ERK phosphorylation is important for inducing cell mobility and tube formation.
7) Phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and Wnt/β-catenin signaling is crucial for cell mobility and tube formation of colon cancer cells, and β-arrs control this signaling that is related to cancer metastasis and progression.
8)
Unvascularized MI, even in well-vascularized MI, would cause certain extent of myocardial damage, and secondary myocardial remodeling, it provokes ischemic HF. In this situation, GPCR and β-arr have important roles in HF pathogenesis because of their canonical β-adrenergic receptor signaling, and CPC starts proliferating and migrating to recover damaged myocardium. Non-canonical and GPCR-independent molecular mechanisms of β-arr may also be important for survival, mobility, and tube formation by CPCs.
9) In these series of adaptation pathways in ischemic HF, we speculate that β-arr has a special role in the migration, proliferation, and tube formation of CPCs, especially in a hypoxic condition, which is similar to acute or chronic myocardial ischemia in vivo.
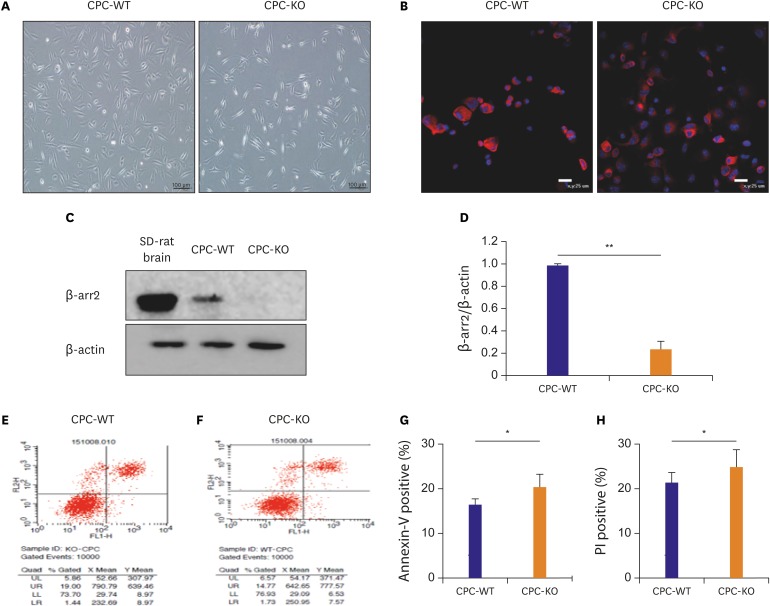
But, there is no specific study about roles and molecular mechanisms of β-arr in CPC under serum deprivation or prolonged hypoxia. With this background, this study was designed to find out the effects of β-arr2 in CPC survival, mobility, and tube formation, and the molecular mechanisms related to these actions, by using wild-type (WT) mouse CPC (CPC-WT) and β-arr2 knock-out (KO) mouse CPC (CPC-KO) in both normoxic and hypoxic cultures to simulate the normoxic and ischemic myocardium.
DISCUSSION
The heart is composed of various types of cells, such as CMC, VSMC, EC, cardiac fibroblasts, and immune cells.
16) However, there are other resident cells in the heart. CPCs comprise less than 1% of the non-CMC population in the heart, and have been sub-classified into c-kit, Sca-1, and Isl-1 positive cells, according to their patterns of cell-surface markers.
4) Among them, c-kit positive CPCs can self-renew, be pluripotent, and differentiate into CMC, VSMCs, or ECs in suitable conditions.
4) Therefore, CPCs can regenerate damaged myocardium by means of CMC replacement and new vessel constitution in MI or chronic myocardial ischemia.
4) Mobilization and active proliferation of CPC in the model of mouse or rat MI have already been elucidated, and injection of
ex vivo expansion CPC effectively repaired left ventricular systolic dysfunction in a rat MI model.
4) These favorable effects of CPC in murine ischemic cardiomyopathy was consistently reproduced in feline and swine models of MI.
17) A significant increase of c-kit positive cells was observed in a human failing heart also.
10) It means that the failing heart can be a cue to start proliferation of nascent CPC to regenerate myocardium.
10) CPCs defined with c-kit positive population from human myocardial tissue expands well in DMEM/F12-based culture medium, and a certain proportion of them proved to have ex vivo expansion capability and differentiation ability to CMC.
11) With such promising data, as an alternative cell source for ischemic HF stem-cell therapy, clinical trials were conducted recently.
4) Makkar et al.
18) injected CPCs as a form of cardiosphere in a failing human heart by an intracoronary route; the whole injection procedure was safe, and the rescuing of HF in the CPC-injected group was evident. van Berlo et al.
19) have shown that endogenous CPC produced new CMC within the heart although at a percentage of approximately 0.03 or less, and CPC amply generated cardiac EC effectively. Cell migration is a critical process for intrauterine fetal development and tissue recovery in various organs.
5) It requires several spatially regulated events involving rearrangement of the actin cytoskeleton, formation of a leading edge, and contraction of the cell cortex.
7)
β-arrs are versatile adaptor and scaffold proteins related to GPCRs.
6) They play key roles in the various receptor functions by acting as either desensitizing and internalizing GPCRs or signal transducers by coupling activated GPCRs.
6) β-arrs are ubiquitous in mammalian cells, but strong expression of β-arrs is observed especially in the brain and spleen.
20) β-arrs are involved in cell proliferation, survival, and mobility, and they control numerous metabolic signaling pathways independently from the canonical GPCR action.
21) β-arrs are important regulators of actin and cytoskeleton reorganization, and are closely related to cell migration.
7) In addition, splenocytes derived from β-arr2 KO mice showed reduced CXCL12-induced migration.
22) Those results indicate that β-arr2 affects cell migration through various molecular pathways.
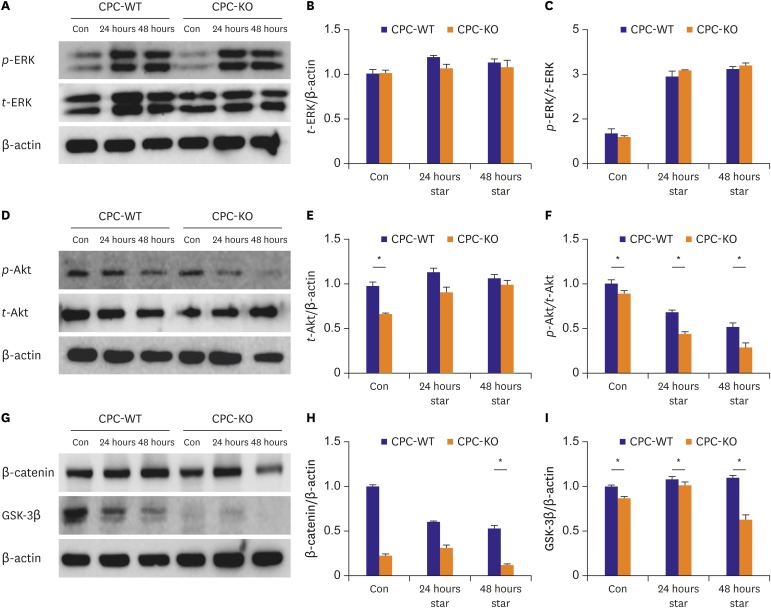
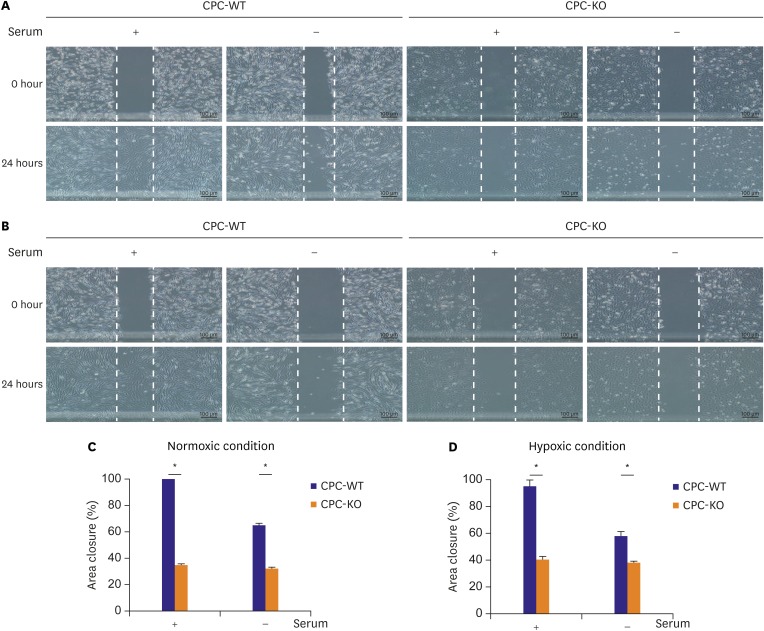
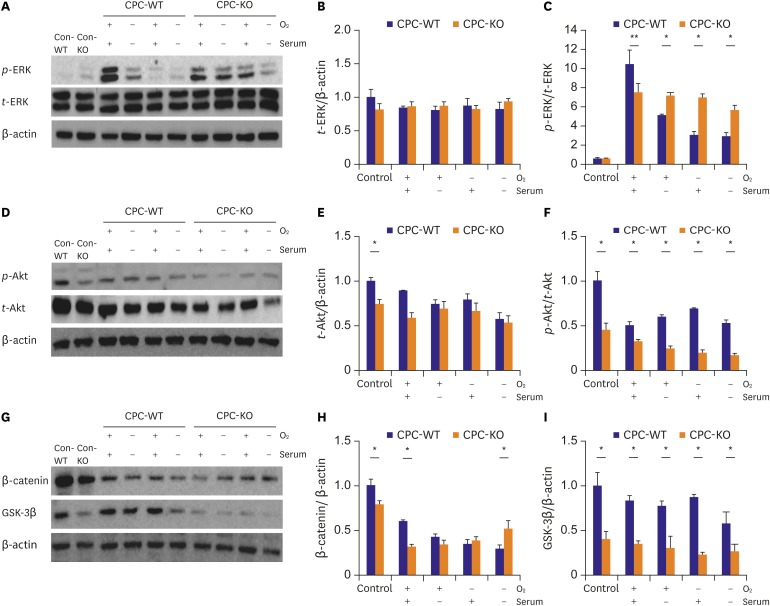
In this study, we demonstrated that CPC-KO healed scratched wound significantly slower than did CPC-WT for all 4 combinations of oxygen concentration and serum supply in culture media (
Figure 3). In the Western blot analysis,
t-ERK showed similar expression in CPC-WT and CPC-KO for the all culture conditions (
Figure 4A and B).
p-ERK/
t-ERK expression was also similar in both CPC-WT and CPC-KO in regular culture condition (
Figure 4A and C). However, CPC-WT showed stronger expression of
p-ERK/
t-ERK than did CPC-KO for the O
2+/serum
+ culture condition, but CPC-KO showed stronger
p-ERK/
t-ERK expression in the other culture conditions (
Figure 4A and C). In the wound healing assay, CPC-WT showed faster migration capability compared to CPC-KO in all conditions. Ge et al.
23) have shown that phosphorylated ERK1/2 causes cell migration in NIH3T3 cells, one type of fibroblast. Our results are opposite to theirs; however, the role of interaction between ERK1/2 and β-arr2 in CPC migration has not been clearly demonstrated and it needs further research to discover about the direct association between β-arr2 and EKR1/2 in migration of CPC. We can neither explain the exact roles of ERK in CPC migration, nor the patterning of contradictory expression of
p-ERK between O
2+/serum
+ condition and the other condition. It needs more detailed experiments and researches about the expression pattern and role of ERK in CPC.
Proteins in Wnt family bind to GPCRs known as frizzled receptors. β-arr2 is an important mediator in the Wnt5A-induced activation of the frizzled 4 receptor.
24) Accumulation of β-catenin in cytosols is involved in cellular proliferation, survival and motility.
24) We demonstrate that the expression of β-catenin was significantly lower in CPC-KO than CPC-WT in routine culture (
Figure 4G and H). However, in the more harmful culture conditions, the β-catenin expression of CPC-KO gradually increased, while that of CPC-WT decreased (
Figure 4G and H). Recent reports have mentioned that β-arr2 KO has been related to lower expression of β-catenin than in the presence of β-arr2.
25) GSK-3β interacts with various pathways, such as Wnt/β-catenin, PI3-K/Akt/mTORC1, and Ras/Raf/MEK/ERK.
24) The GSK-3 gene consists of GSK-3α and GSK-3β. GSK-3β is phosphorylated by Akt, and then GSK-3β is inactivated.
24) Our results showed that CPC-WT expressed a significantly higher amount of GSK-3β in all cultures than CPC-KO did (
Figures 2G,
2I,
4G, and
4I). Akt activation plays important roles in many cellular functions, such as survival, proliferation, migration, and tube formation. Deactivation of Akt stimulates GSK-3β and increases cell activity.
24)26) These results showed lower expression of
p-Akt in CPC-KO than CPC-WT in all cultures (
Figures 2D,
2F,
4D, and
4F). Cardiac tube formation or angiogenesis is important for delivering oxygen, various growth factors, cytokines, and chemokines to damaged regions.
27) Also, β-arrs are a main regulator of tumor angiogenesis,
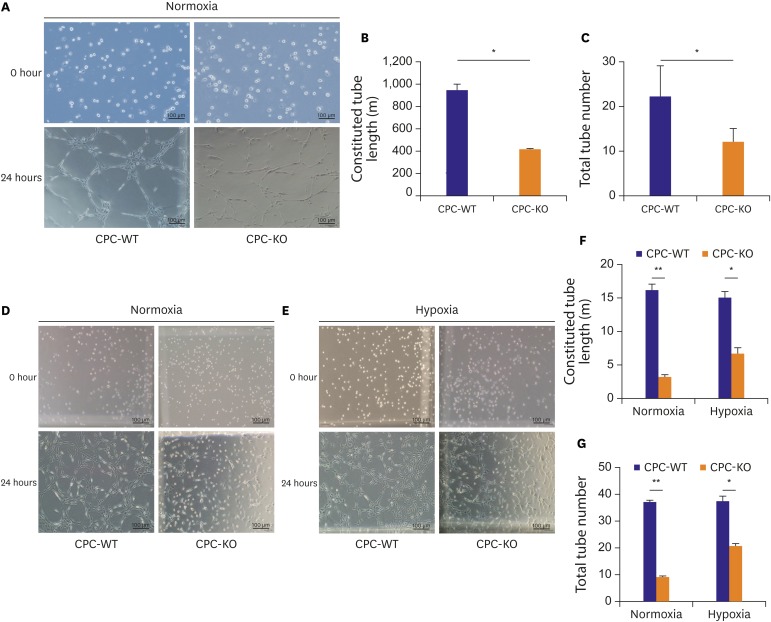
28) but the role of β-arrs in CPC is not clear. Our results showed that CPC-WT generated more complete tubes than CPC-KO in all cultures (
Figure 5). Under pre-serum starvation, CPC-WT and CPC-KO both increased the tube length and number of complete tubes more than they did without pre-serum starvation (
Figure 5A-C). Hypoxia generally provokes rapid tube formation in mesenchymal stem cell family compared to normoxic condition.
29) CPC-KO showed more constitution of tube in hypoxia compared to normoxia, but this common phenomenon was not reproduced in CPC-WT in
Figure 5. We surmise that CPC-WT showed relatively fast tube formation compared to previous data based on bone marrow derived mesenchymal stem cell or adipose tissue derived stem cell, so hypoxia did not have great affection to formation of tube in CPC-WT compared to CPC-KO.
30) This point should be confirmed by comparative experiment using CPC, mesenchymal stem cell and adipose derived stem cell in same culture media under stimulating condition. Molecular pathway exploit about tube formation of CPC-WT in hypoxia is new research target in our laboratory. With above our data about β-arr2 actions in CPC, we surmise that β-arr2 will be very important scaffolding protein beside of canonical action in GPCR to make CPC migration, proliferation, and new vessel formation in MI inducing advanced HF. This hypothesis should be examined in MI model in β-arr2 KO mouse. There are some limitations in our data. First of all, we did not perform experiments with antagonist for effector molecules, e.g., Akt, GSK-3β, or β-catenin on CPC mobility, tube formation or survival on serum deprivation or hypoxemia. So, we could not make direct interaction between β-arr2 and tentative effector molecules in CPC performances. Second, the role of ERK in CPC was very ambiguous. According to previous data, ERK showed contradictory roles in cell mobility and survival in various cell types.
7)8)
p-ERK expression patterns in serum deprivation conditions were very ambiguous in CPC, and it was very difficult to estimate the role of ERK in CPC. We thought that it must be considered to do fine experiments to know the exact roles of effector molecules in CPC.
In summary, β-arr2 deficiency reduced mobility and tube formation ability of CPC in the presence or absence of serum and oxygen. It would be related to CPC survival given hypoxia or serum deprivation. Therefore, β-arr2 deficiency may be related to delayed myocardial regeneration via CPC mobility and tube formation disorder in ischemia.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download