This article has been
cited by other articles in ScienceCentral.
Abstract
It has been estimated that coronary chronic total occlusion (CTO) is encountered in 15 to 20% patients referred for coronary angiography (CAG). The success of percutaneous coronary intervention (PCI) of CTO can be attributed to the vast array of hardware that has now become available and also to the vastly enhanced operator expertise. It is however realistic to state that despite the tremendous increase in the rate of success, there then comes a subset of CTO where PCI attempts fail. The reason for such failures given that other variables remain constant is the inability to cross the CTO lesion. This can be due to a failure to cross the lesion with a guide wire (despite guide wire escalation). The second cause of failure is the inability to cross the lesion with a balloon (balloon-uncrossable [BU] CTO). This can occur despite the successful placement of a guidewire in the distal true lumen. The BU lesions contribute 2% to 10% of CTO PCI failure cases. The author attempts to present a creative solution to assist crossing such lesions.
Keywords: Chronic total occlusion, Percutaneous coronary intervention, Balloon-uncrossable
INTRODUCTION
Inability to cross the lesion with a guidewire is the most common reason for failure of percutaneous coronary intervention (PCI) of chronic total occlusion (CTO).
1) Failure to cross the CTO after successful guidewire crossing is the second most common cause for CTO PCI failure, occurring in up to 10% of cases.
2)3) The balloon-uncrossable (BU) lesion is often due to severe calcification at the occlusion site or a significant tortuosity or a combination of both. The incidence of such BU CTO is not uncommon even after rapid advances in technique and technology. The author outlines a step-by-step approach for such lesions.
AUGMENTATION OF GUIDE CATHETER SUPPORT
Good guide catheter support is required for BU CTO, and this is the basis for selecting a guide catheter. It has been found that the catheter size, contact area, and angle on the reverse side of the aorta play significant role in influencing the backup support of guiding catheters. Guide catheter support can be advanced using larger size catheter, deep throating (active support), passive support, buddy wires, guide catheter extensions, and side-branch anchor techniques (
Table 1).
Table 1
Overcoming BU CTO

|
• Augmented guide catheter support |
|
Larger guide catheter with more supportive shape |
|
Long arterial sheaths |
|
Deep engagement |
|
Guide catheter extension |
|
Anchor wire |
|
Buddy wire |
|
Anchor balloon: side branch, distal target vessel or subintimal at or below lesion site |
|
• Lesion modification |
|
Appropriate small balloon (1.20–1.5 mm) manipulation |
|
Wedgies & grenadoplasty (Intentional balloon rupture) |
|
Microcatheters: Tornus, Corsiar, Carvel, Finecross, Turnpike |
|
Excimer laser: ablative and acoustic energy |
|
Rotational atherectomy |
|
Seesaw balloon-wire cutting technique |
|
Multi-wire plaque crushing technique |
|
Crowbar effect |
|
Retrograde approach |
Size of guiding catheter
As the size of the guide catheter increases, the backup support provided increases significantly. It is often possible to successfully complete PCI by simply upgrading the size of the catheter without additional maneuvers. However, the stiffness and support increases the risk of ostial coronary dissection. Currently 6–7 F catheters have internal lumens that are large enough as previous generation 8 F catheters.
Active guide support
Active guide support is usually obtained by operator-dependent techniques, such as deep seating of the guide beyond the ostium or by molding the catheter in the ostium. Deep guide catheter intubation can significantly
4) increase back up support although it carries a minor risk of ostial dissection. Active support could be achieved by rotation for right coronary artery and forward pushing for left main. Active back up catheters (such as JL) have softer tips and a shape that can be modified to accommodate aortic root measurements by active manipulation with the tip position adjusted to achieve a stable coaxial position. As the size of the catheter increases, the role of active support decreases.
Passive guide support
Passive guide support can be achieved by larger caliber guide catheter (7–8 F) or more supportive shapes (such as Amplatz, XB or EBU guide catheter). The inherent passive support provided by the guide is dependent on the shape of the guide. The latter permits the guide to achieve coaxial alignment with the coronary ostium, and additionally gain support from opposite wall of the aorta.
Buddy wire
One or more buddy wires positioned in the main branch or a side branch augments both stability of guide catheter and support for balloon.
Guidecatheter extensions
Another technique for increasing guide support involves use of mother-child (5-in-6) system. The mechanism of enhanced support of the “child” catheter is change in shape of “mother” catheter so that support from the contralateral aortic wall is obtained and deep intubation of the coronary is possible without complications. The GuideLiner catheter (Vascular Solutions Inc., Maple Grove, MN, USA) is based on the mother-child concept, with a 4 F child catheter intended to provide the extra support needed for balloon to cross. A GuideLiner, Guidezilla (Boston Scientific, Natick, MA, USA) or Guidion (IMDS, NZ Roden, Netherlands) or Heartrail (Terumo, Kanagawa, Japan) catheter is advanced into the artery, augmenting guide catheter support and pushability of balloons/microcatheters (
Figures 1 and
2). The Guidezilla catheter is similar to GuideLiner but has slightly larger internal diameter (1.45 vs. 1.42 mm respectively). Use of guide catheter extension can also decrease the amount of contrast injected and provide improved visualization via sub-selective engagement of artery. One must be cautious when advancing this catheter into a coronary artery as these devices may cause ostial or mid target vessel dissection.
5) Kinking of the guide catheter during engagement of the coronary ostium can be avoided by either deep inspiration or leaving the J wire within the catheter to enhance torqueability.
Figure 1
Algorithm for crossing a BU CTO.
BU = balloon-uncrossable; CTO = chronic total occlusion.
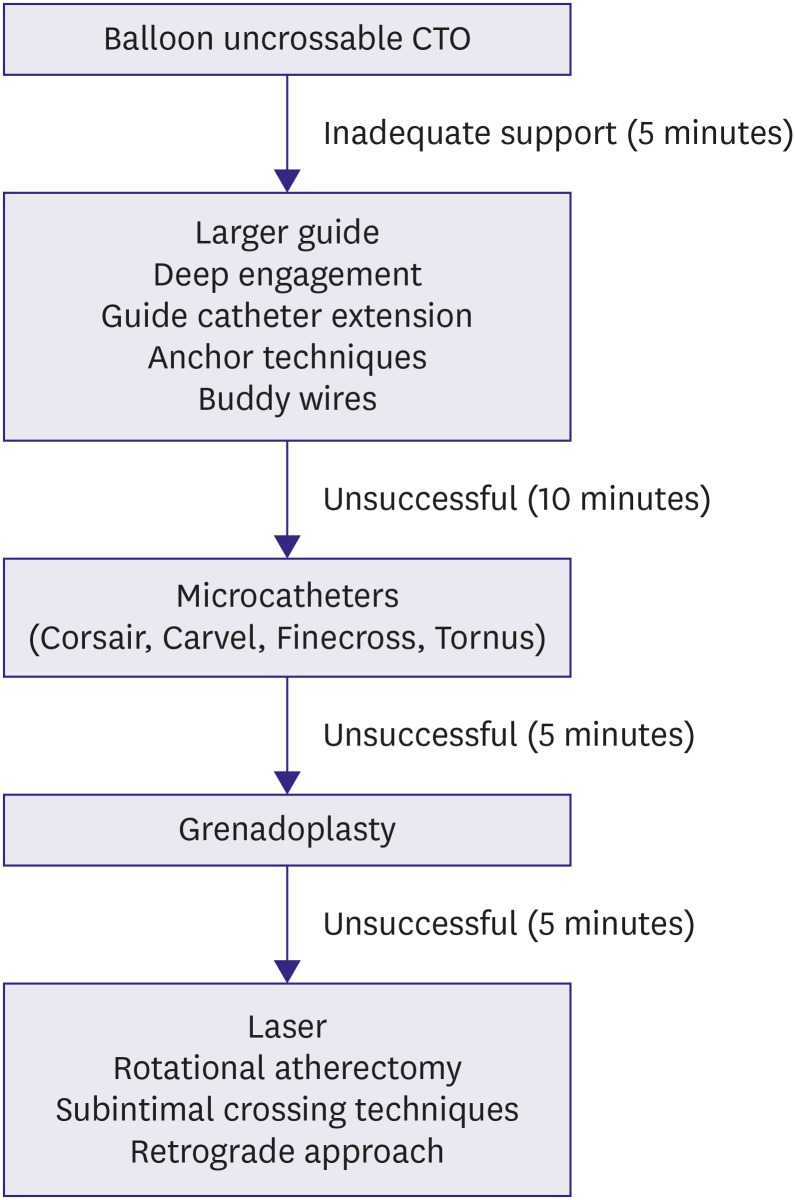
Figure 2
Schematic illustration of augmentation of guide catheter back up by guide extension catheter.

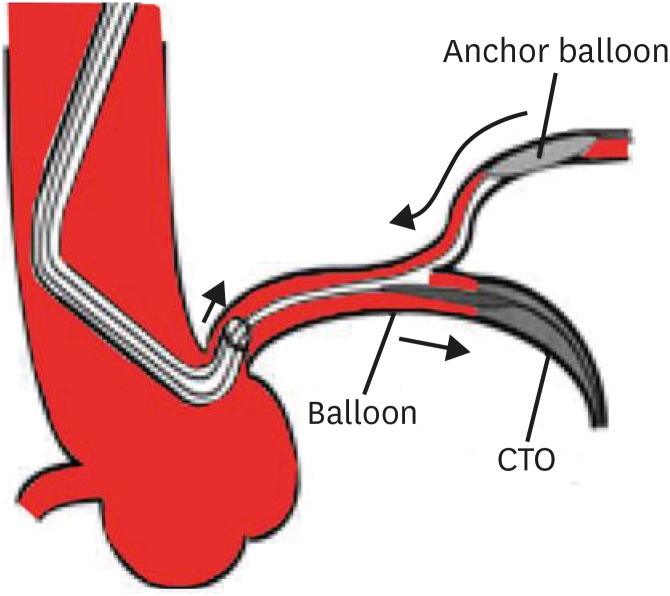
Side-branch anchor technique
A workhorse guidewire is negotiated into a side branch (usually a conus or acute marginal branch for the right coronary artery [RCA] or a diagonal or obtuse marginal for left circumflex or the left anterior descending [LAD] artery), followed by a small balloon (1.5–2.0 mm) inflation at 6–8 atm that anchors the guide catheter into the vessel and facilitates advancement of balloon or microcatheters (
Figures 3 and
4). It should be that care should be taken not to damage the side branch. If a previously inserted stent is present, expanding the balloon from the inside of the stent can perform balloon anchoring without worrying about vessel damage.
Figure 3
(A) Engagement of LMCA ostium with 6 F EBU 3.5 guide catheter to address long mid segment CTO of LAD artery. (B) Failure of 1.25×12 mm balloon to cross the lesion after insertion of Gaia 2 guidewire followed by navigation of 2.1 F Tornus catheter. (C) A 2×12 mm balloon in diagonal artery acts as an anchor. (D) Final result after deployment of 2 overlapping DES.
CTO = chronic total occlusion; DES = drug-eluting stent; LAD = left anterior descending; LMCA = left main coronary artery.
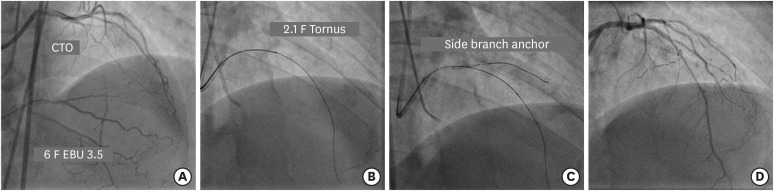
Figure 4
Schematic illustration of side branch anchor balloon technique.
CTO = chronic total occlusion.
APPROPRIATE BALLOON MANIPULATION
After a guide catheter is firmly engaged into the coronary artery, the single marker rapid exchange low-crossing profile balloon (1.2–1.5 mm in diameter) with long length (20–30 mm) is pushed against the lesion with alternating forward and backward movements. This procedure utilizes the physical principle that dynamic friction is smaller than static friction. Balloon passage is also assisted by deep inspiration causing stretch of guide catheter and the balloon shaft. Once the balloon stops advancing, it can be inflated up to approximately 12 atm while maintaining forward pressure. This may crack the proximal cap to allow lesion crossing, sometimes even with the same balloon. If it does not advance further after inflation, the operator should try with a new small balloon, or one manufactured by another company, as different crossing profile and tip may assist in balloon passage across CTO. Sometimes inflation with a larger diameter balloon (2.5–3.0 mm) just proximal to CTO lesion modifies proximal cap of CTO enough to allow subsequent crossing of a small profile balloon.
MICROCATHETER ADVANCEMENT
Successful navigation of a specialty microcatheter (Tornus, Corsair, Caravel [Asahi Intecc, Nagoya, Japan], Finecross [Terumo], Turnpike [Vascular Solutions Inc.], MultiCross and CenterCross [Roxwood Medical, Inc., Redwood City, CA, USA]) could cross the BU lesion or assist the balloon to pass through the lesion. Alternatively, the guidewire could be exchanged for a rotational atherectomy wire, if debulking is planned as the next step.
The Tornus (Asahi Intecc) is a special microcatheter (
Table 1) for penetrating hard BU lesion (
Figure 5) which has a 1 mm blunt tip that is composed of platinum and eight bundles of stainless steel wire extending from the tip. The stainless-steel wire acts like a screw against the vessel wall. Its likelihood of crossing BU lesions is attributed to very strong driving force generated by counterclockwise rotation against the arterial wall and non-bending nature unlike balloon.
6)7) With the extension wire attached to stiff wire that has crossed CTO, the Tornus is advanced to a point immediately proximal to the occlusion. After gripping the torquer with the 4th or 5th fingers of right hand, the is advanced by counterclockwise rotation with the first to third fingers taking utmost care to avoid distal perforation by unexpected movement. Over torquing may damage the device by disruption of stainless steel wire bundles leading to catheter entrapment or shaft breakage. The author recommends that the counterclockwise rotation should not exceed 20 revolutions in the 2.6 F Tornus and 40 turns in 2.1 F device before allowing the catheter to unwind. Once it gets stuck up in CTO, the accumulated torque must be released before the upper limit of rotations is reached. Clockwise rotation (20 revolutions in the 2.6 F Tornus and 40 revolutions in 2.1 F) should be applied when the Tornus is being removed.
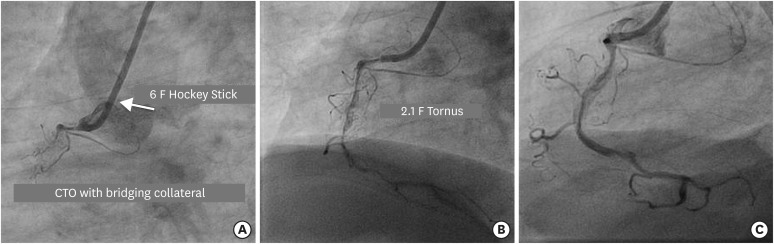
Figure 5
(A) Engagement of RCA ostium with 6 F Hockey Stick 4.0 guide catheter to address long segment CTO of ostio-proximal segment with bridging collateral. (B) Navigation of 2.1 F Tornus (in BU lesion) after Gaia 3 guidewire penetrates the proximal cap to be positioned in the distal true lumen. (C) Final result after spot stenting in long calcified lesion.
BU = balloon-uncrossable; CTO = chronic total occlusion; RCA = right coronary artery.
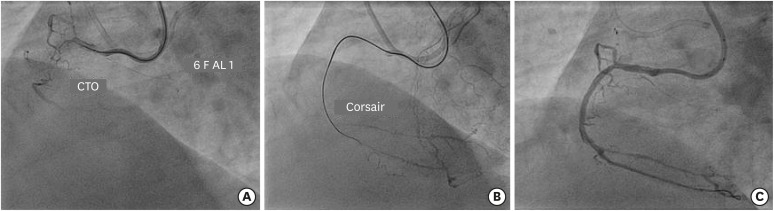
The 135-cm long Corsair and Caravel (Asahi Intecc) can be advanced by rotating in either direction, although they are braided to have better penetration power when rotated counterclockwise (
Figure 6). In case of further resistance, counterclockwise rotation along with forward push is the most powerful maneuver. The hydrophilic coated tapered soft tip of Corsair provides superior tip flexibility and maneuverability and the shinka shaft with braiding pattern provides unique pushability, trackability, and support. Over rotation of this device (>10 revolutions) should be avoided as it could cause catheter deformation, entrapment or fracture proximal to the catheter tip. Excessive manipulation of the Corsair may cause disruption of the device, wire and lock both requiring withdrawal.
Figure 6
(A) Engagement of RCA ostium with 6 F AL 1guide catheter to address CTO of proximal segment with bridging collateral. (B) Facilitation of balloon crossing by Corsair microcatheter after Gaia 2 guidewire crosses the lesion. (C) Final result after deployment of 2 overlapping DES.
CTO = chronic total occlusion; DES = drug-eluting stent; RCA = right coronary artery.
MultiCross and CenterCross (Roxwood Medical, Inc.) catheters utilize an atraumatic nitinol anchoring mechanism to simplify pass across BU CTO.
INTENTIONAL BALLOON RUPTURE
A small (usually 1.20–1.5 mm) Threader or Glider balloon (both manufactured by Boston Scientific) is negotiated as far as possible into the lesion and inflated at high pressure until it ruptures. This balloon-assisted microdissection (grenadoplasty) or intentional balloon rupture can modify the plaque leading to successful cross with another balloon (
Figure 1). It may lead to vessel dissection and perforation and sometimes there may be difficulty in removing the ruptured balloon.
EXCIMER LASER CORONARY ATHERECTOMY (ELCA)
Excimer lasers are pulsed gas lasers using a mixture of a rare gas and halogen as the active medium to generate pulses of short-wavelength, high-energy ultraviolet light. The 0.9-mm ELCA (point 9; Spectranetics Corporation, Colorado Springs, CO, USA) is used at maximum repetition and fluence levels (repetition rate Hz and fluence of 80 mJ/mm
2).
8) This may modify the proximal cap facilitating balloon entry into the lesion.
9) The point 9 xenon chloride excimer laser catheter is a pulsed ultraviolet laser catheter. It causes minimal thermal injury to tissues and is capable of ablating calcified plaque as compared to longer-wavelength continuous-wave lasers.
10) Flushing with saline is recommended during ELCA catheter navigation. It should be performed once it is confirmed that PCI guidewire is present in the true-lumen to avoid vessel perforation.
PERCUTANEOUS CORONARY ROTATIONAL ATHERECTOMY (PTCRA)
PTCRA with a small diameter burr (1.25–1.50 mm
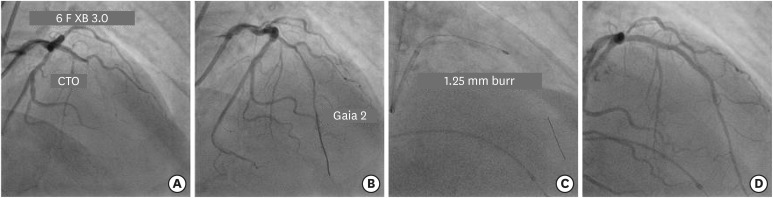
) renders differential ablation of calcific tissue in CTO that can greatly facilitate lesion crossing with a balloon (
Figure 7). However, it requires exchange for dedicated 0.009" rotawire (Boston Scientific), which may not be feasible in CTO cases.
2) After an over-the-wire microcatheter is advanced as far as possible into the CTO lesion, the stiffer CTO wire is exchanged for a rotawire. The burr should be advanced in a repetitive and gentle manner avoiding forceful “wedging” into the occlusion.
Figure 7
(A) Engagement of LMCA ostium with 6 F XB 3.0 guide catheter to fix long mid segment CTO of LAD. (B) Navigation of Gaia 2 guidewire across the occlusion into distal true lumen. (C) Rotational atherectomy with 1.25 mm burr to facilitate lesion crossing with balloon. (D) Final result after 2 overlapping DES.
CTO = chronic total occlusion; DES = drug-eluting stent; LAD = left anterior descending; LMCA = left main coronary artery.
SUBINTIMAL CROSSING TECHNIQUE
A subintimal crossing technique can be used in an attempt to cross the lesion through a path of least resistance. A second guidewire is advanced subintimally distal to the CTO. Alternatively, the CrossBoss catheter (Boston Scientific) is negotiated to achieve subintimal position and exchanged for the guidewire. If the subintimal guidewire-navigates into distal true lumen, it can be used for angioplasty and stenting instead of the original wire. A balloon is inflated over the subintimal guidewire next to the CTO crushing the CTO plaque from the outside. However, this technique has limitations, such as occasional difficulty in re-entering the distal true lumen, which can be facilitated by use of dedicated equipment (stingray balloon and guidewire [Boston Scientific]).
11)
SUBINTIMAL DISTAL ANCHOR TECHNIQUE
A second guidewire is advanced subintimally distal to the CTO. The balloon is advanced over the same wire distal to the CTO and is inflated anchoring the true guidewire placed in true lumen, and rendering antegrade delivery of a balloon over the true lumen guidewire.
12)
MULTI-WIRE PLAQUE CRUSHING TECHNIQUE
Multi-wire plaque crushing technique is one of among all which had also proven effectively. The multi-wire plaque crushing technique is to insert 1–2 wires along with the original wire located in the true lumen of CTO after balloon failure for plaque crushing and then to withdraw the crushing wires to get an enlarged lumen inside the occlusion segment, thus facilitating the passing of the low-profile balloon.
13)
SEASAW BALLOON-WIRE CUTTING TECHNIQUE
The seesaw balloon-wire cutting technique is 1 of the effective and safe technique to facilitate balloon crossing during CTO interventions. The main process of this technique is to insert 2 guide wires (guide wires A and B) into the distal true lumen of CTOs and then to advance 2 short and low-profile balloons (balloons A and B) over the 2 guide wires, respectively. Balloon A is first advanced over guide wire A as distally as possible, and then is inflated with high pressure (≥18 atm) to press guide wire B, producing a cutting power to crush the proximal fibrous cap of the CTO. Subsequently, balloon A is withdrawn slightly, and balloon B is advanced as distally as possible and then is inflated to press guide wire A, producing a similar cutting effect to crush the proximal fibrous cap on the other side. The 2 balloons are progressed alternatively until 1 of them is able to cross through the occluded segment.
14)
CROWBAR EFECT TECHNIQUE
“Crowbar effect” is an alternative antegrade method for opening BU CTO. This procedure is easy to accomplish and success rates are high. The fluoroscopy time and contrast consumption are less. In this approach a soft guidewire (i.e. Fielder XT, XTA, Gaia 1; Asahi Intecc) is inserted though the CTO lesion which is followed by insertion of second (i.e. Miracle 3, 6, Gaia 2; Asahi Intecc) and third stiffer guidewire (i.e. Miracle 12, Conquest Pro 12, Gaia 2, Gaia 3; Asahi Intecc) along the trace of the first guidewire into distal vessel true lumen. Then a small balloon of 1.25–1.5 mm is placed into the lesion by repeatedly inflating the balloon with high pressure (14–16 atm) which moves the balloon forward 2–3 mm with each inflation until it crosses the occlusion. Then second and third guidewires are withdrawn and bigger balloon with a diameter of 2.0 mm or larger is inserted along the retained soft guidewire. The created channel is inflated further with the balloon and stenting is performed.
15)
RETROGRADE APPROACH
Many cases of BU CTOs can be managed by the retrograde approach that involves both antegrade accesses to the proximal cap, retrograde access to the distal cap, creation of subintimal space by antegrade balloon followed by negotiation of retrograde wire through the subintimal space into the proximal true lumen. Adoption of this technique has potentially improved the success rate of complex CTO PCI to the extent of 90–95%.
16)17)18) The retrograde approach should be used a ‘last resort’ effort to treat BU CTO as it requires: appropriate interventional collaterals, local expertize, dedicated equipment. It increases radiation exposure, contrast limits and has inherent risks such as donor vessel trouble (thrombus, dissection) and collateral vessel perforation. However, the fear of complications should never deter an interventionist to perform this procedure if it is significantly beneficial to the patient. This retrograde or backdoor should not be locked in spite of some catastrophic complications most of which are preventable.
19)
CONCLUSION
Successful CTO PCI often requires flexibility and creativity as each CTO is unique. The second most common cause of CTO PCI failure is BU lesions in spite of successful wire positioning in the distal true lumen. The calcification and tortuosity at the lesion site primarily accounts for it. The main principle behind to achieve success in CTO PCI of such lesions is to have a strategy for good guide catheter backup support. Once strong back up support is obtained and there still remains a difficulty in crossing the lesion, lesion modification should be considered. The simultaneous and sequential applications of various techniques can enhance the likelihood of successful balloon crossing.









 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download