Abstract
Background and Objectives
Non-calcified carotid plaques are more unstable than calcified plaques, and they are associated with a higher risk of rupture, thromboembolism, and consequently, stroke. The purpose of the present study is to compare calcified and non-calcified plaques that cause intermediate carotid artery stenosis with respect to neutrophil/lymphocyte ratio (NLR).
Subjects and Methods
A total number of 139 asymptomatic patients with 50-70% stenosis of the carotid artery were included in this study. Carotid Doppler ultrasound imaging and computed tomography angiography were performed to divide the carotid artery plaques into two groups as calcified and non-calcified. Patients included in the calcified (n=73) and non-calcified (n=66) plaque groups were compared with respect to total neutrophil count, lymphocyte count and NLR.
Results
Total lymphocyte count was statistically significantly lower in the non-calcified plaque group compared to the calcified plaque group (total lymphocyte count in non-calcified/calcified plaque groups [103/mm3]: 2.1/2.3, respectively) (p=0.002). NLR was statistically significantly higher in the non-calcified plaque group compared to the calcified plaque group (NLR in non-calcified/calcified plaque groups: 2.6/2.1, respectively) (p<0.001). The cut-off value for NLR was found to be >2.54. Multivariate regression analysis showed that NLR was independently associated with non-calcified carotid artery plaques (odds ratio 5.686, 95% CI 2.498-12.944, p<0.001).
Atherosclerotic lesions are the leading cause of ischemic stroke worldwide.1) The primary factor in the pathogenesis of atherosclerosis is inflammation. Development and progression of an atherosclerotic plaque is mainly regulated by inflammatory and immune mechanisms.2)
The risk of rupture of atherosclerotic plaques is more serious than the severity of stenosis caused by plaques.3) Detection of early neutrophil infiltration in the atherosclerotic plaques allows prediction of the risk of plaque rupture.4) Histopathological examinations of carotid artery plaques demonstrated that the rupture-prone lesions are associated with higher neutrophil count.5) In a study investigating the carotid endarterectomy (CEA) specimens, coexistence of neutrophil infiltration and hemorrhage into the plaque was demonstrated particularly in the culprit zone of the carotid plaque.6) Previous studies have shown that lymphopenia is one of the early markers that develop secondary to stress-related cortisol release in patients who have experienced a myocardial infarction.7) There is a negative correlation between lymphocyte count and cardiovascular prognosis.8)9) Currently, neutrophil/lymphocyte ratio (NLR) is considered to be a good marker that simultaneously shows the negative effects of neutrophil elevation as an indicator of acute inflammation and lymphocyte depletion as an indicator of physiological stress, as well as it helps prediction of mortality and prognosis of stroke patients.10)11)
Plaque morphology is an independent risk factor for prognosis in coronary artery disease patients. Non-calcified plaques are associated with worse clinical outcomes. After 78-months of follow-up, mortality rate among patients with calcified coronary plaques, mixed plaques and non-calcified plaques was shown to be 1.4%, 3.3% and 9.6%, respectively.12)13) Coronary computed tomography angiography can provide reliable information on plaque morphology.14)
Embolism and stroke risks associated with calcified and non-calcified plaques in the carotid artery are different. Histopathological examinations performed after CEA have demonstrated that calcified plaques are more stable, and they carry a lower risk of rupture and thromboembolism.15) On the other hand, non-calcified and ulcerous plaques are more fragile and they carry a higher risk of rupture; therefore they are associated with increased risk of embolism and stroke.16)
Severity of a carotid artery lesion is an important parameter that affects the risk of stroke. The risk of stroke increases with the severity of carotid artery lesion.17) In order to perform carotid artery intervention, the level of stenosis should angiographically be at least 50% in symptomatic patients and at least 70% in asymptomatic patients.18) In symptomatic patients, a plaque that causes 50% stenosis in the carotid artery may pose a risk of recurrent stroke and requires surgical intervention or placement of a stent. There is currently no test that can be used to predict the risk of stroke in asymptomatic patients with intermediate (50-70%) carotid artery stenosis. We believe that non-calcified plaques that cause asymptomatic intermediate carotid artery stenosis are more active in terms of inflammation and subsequent atherothrombosis; therefore they may be associated with a higher risk of stroke than calcified plaques. The present study compares calcified and non-calcified plaques that cause asymptomatic intermediate carotid artery stenosis with respect to total neutrophil count, lymphocyte count and NLR.
A total number of 164 patients were evaluated for enrollment in this prospective study. Twenty-five patients were excluded from the study due to various reasons and the analyses were performed on the data obtained from 139 patients (Fig. 1). The patients were divided into two groups, as those having calcified (73 patients) and non-calcified (66 patients) plaques. The study was approved by the ethics committee of our institution. Informed consent was taken from all patients. Patients with 50-70% stenosis of the carotid artery were included in the study. Initially carotid Doppler ultrasound (CDUS) and then computed tomography angiography (CTA) were performed in all patients. The study cohort consisted of patients who had been assessed by a team that included a neurologist, a cardiologist, a radiologist and a cardiovascular surgeon and who had been confirmed to be asymptomatic with respect to the carotid artery lesion. Symptomatic patients were defined as those who experienced an ischemic cerebrovascular event with or without a sequel, a transient ischemic attack or amaurosis fugax within the last 6 months, and they were excluded from the study. Patients with diabetes mellitus were examined by an endocrinologist and they were included in the study after their blood glucose levels had been regulated. Patients were included in the study at least two months after initiation of antiaggregant, antihyperlipidemic and antihypertensive therapies. Blood samples were obtained at two different time points, 15 days apart. Laboratory parameters that have been analyzed in this study represent the mean value of two measurements taken at different time points by using the same device. Patients with a history of ischemic or non-ischemic stroke, systemic inflammatory disease, cancer, acute coronary syndrome, previous myocardial infarction, heart failure, significant valvular disease, chronic obstructive pulmonary disease, renal or liver failure, an hematological disease; patients whose body temperature was ≥37℃; patients who had an active infection, white blood cell count (WBC) >12.000 per µL; and patients who were using anti-inflammatory drugs or antibiotics were excluded from the study.
Fasting blood samples were drawn from a large antecubital vein of each patient for determination of biochemical and haemostatic parameters. EDTA-tubes were used for automatic blood count. The blood counts were measured by a Beckman Coulter LH 780 Hematology Analyzer. Total cholesterol, low-density lipoprotein, triglyceride and high-density lipoprotein levels were measured by colorimetric method (Abbott Laboratories, Abbott Park, IL, USA). Baseline NLR was calculated by dividing neutrophil count to lymphocyte count.
Hypertension was defined as a systolic/diastolic blood pressure of ≥ 140/90 mmHg; or patients using antihypertensive medications. Diabetes mellitus was defined as a fasting plasma glucose level of ≥126 mg/dL or patients actively using oral antidiabetics and/or insulin. Patients who smoked regularly were considered as smokers. Hyperlipidemia was defined as a total cholesterol level of ≥200 mg/dL. Coronary artery disease was defined angiographically as the presence of a plaque resulting in 50% or more stenosis in a major coronary artery. Body mass index (BMI) was calculated by dividing the body weight (kg) to the square of the height (m).
Carotid artery stenosis was first assessed by CDUS and then by CTA. CDUS examination was performed using Esaote s.p.a MyLabClass C (Florence-Italy) device and a linear arrayed probe that allows selection of frequencies between 3-11 mHz. CTA was performed using a CT device with the Philips Brilliance 64 detector (Holland). After venous access was established through the antecubital vein and 80 mL non-ionic contrast agent was administered at a rate of 4.5 mL/sec, axial-plane CT images of the carotid and cerebral arteries were obtained using the tracking method. Acquired slices were transferred to the work-station (Philips Intellispace Portal) and multi-plane images, maximum intensity projection and volume rendering 3-dimensional images were developed by post-processing the original slices via appropriate software (AVA). These images were reviewed with respect to vascular plaques and stenosis.
The stenosis caused by plaques as observed using CDUS was assessed according to the criteria developed by the internal carotid artery stenosis criteria consensus committee. Internal carotid artery plaque characteristics were evaluated by a 64 slice CT and plaque burden was evaluated according to the thickness of the plaque. The measurement of the stenotic arterial lumen was performed where it was the narrowest and in the thicker region of the plaque. The level of stenosis based on CTA was evaluated according to the NASCET (North American Symptomatic Carotid Endarterectomy Trial) criteria. Based on the CDUS and CTA examinations, plaques that were fully soft and calcified less than 50% were categorized as non-calcified plaques whereas those that were fully hard and calcified more than 50% were categorized as calcified plaques. In other words, hyperdense plaques (containing more than 50% calcific component and a measured plaque density of more than 120 Hounsfield units [HU]) were regarded as calcified plaques, and hypodense plaques (containing less than 50% calcific component and a measured plaque density of less than 120 HU) were considered as non-calcified plaques.19)
Data were analyzed by the SPSS software version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). Categorical variables were presented as frequency and percentage. The χ2 test and Fisher's exact test were used to compare categorical variables. The Kolmogorov–Smirnov test was used to assess the distribution of continuous variables. Student's t-test was used for variables with normal distribution and the values were presented as mean±SD. Continuous variables without normal distribution were analyzed using Mann-Whitney U test and obtained values were presented as median (50th) values and interquartile ranges (25th and 75th). Receiver-operating characteristic (ROC) curves were drawn with sensitivity and specificity analyses. Multivariate logistic regression analysis was used to evaluate the independent associates of the risk of non-calcified plaque. The odds ratios (OR) and 95% confidence intervals (CI) were calculated. A two-tailed p value of <0.05 was considered statistically significant.
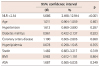
Table 1 shows the demographic and clinical characteristics in addition to laboratory results of 139 patients (73 with calcified plaques, 66 with non-calcified plaques) who were included in the study. No significant difference was noted between the two groups in terms of the baseline parameters.
Table 2 shows the comparison of hematological data between the two groups. Mean hemoglobin level was 13.5±1.5 g/dL in the calcified plaque group and 12.8±1.4 g/dL in the non-calcified plaque group. Calcified plaque group had statistically significantly higher hemoglobin levels (p=0.013). WBC count was 8.0±1.6 103/mm3 in the calcified plaque group and 7.6±1.8 103/mm3 in the non-calcified plaque group; the difference between WBC counts in the two groups was not statistically significant (p=0.667). Mean total neutrophil count in the calcified and non-calcified plaque groups was 4.8 103/mm3 and 5.2 103/mm3, respectively. The increase in total neutrophil count in the non-calcified plaque group did not reach the level of statistical significance (p=0.227). Mean total lymphocyte count in the calcified and non-calcified plaque groups was 2.3 103/mm3 and 2.1 103/mm3, respectively; and the difference between the two groups was statistically significant (p=0.002). NLR was 2.1 in the calcified plaque group and 2.6 in the non-calcified plaque group, and comparison of NLR between the two groups demonstrated that it was statistically significantly higher in the non-calcified plaque group (p<0.001) (Fig. 2).
In ROC curve analysis, an NLR cut-off value of >2.54 was found for calcified-non calcified plaque discrimination among carotid arteries plaques which caused intermediate stenosis (Area Under Curve [AUC]: 0.699, CI 95% 0.611-0.787, p<0.001). NLR of >2.54, had 53% sensitivity and 84% specificity in calcified - non calcified plaque discrimination (Fig. 3).
The multivariate logistic regression analysis including age, hypertension, diabetes mellitus, coronary artery disease, hyperlipidemia, statin use, BMI, acetylsalicylic acid (ASA) use and NLR showed that the NLR was independently associated with non-calcified carotid plaques (OR 5.686, 95% CI 2.498-12.944, p<0.001) (Table 3).
This is the first study comparing calcified and non-calcified plaques that cause asymptomatic intermediate carotid artery stenosis with respect to inflammatory parameters. In the present study, stenosis and plaques in the carotid artery were assessed by CDUS and CTA; and the calcified plaque group was compared with the non-calcified plaque group in terms of total neutrophil count, lymphocyte count and the NLR. The findings of this study indicate that among asymptomatic plaques that cause intermediate carotid artery stenosis, non-calcified plaques are associated with a lower total lymphocyte count and a NLR that is significantly higher than those observed in the presence of calcified plaques.
Atherosclerosis and cardiovascular diseases are the most common causes of mortality worldwide.20) Inflammation plays a role in every stage of atherosclerosis, including its development, progression and rupture of the atherosclerotic plaque.21) Neutrophils have certain effects on plaque rupture, reperfusion damage and remodeling.6) Nasr et al.22) have previously demonstrated an association between neutrophil count and cerebral microembolization in symptomatic patients with carotid artery stenosis. Ionita et al.23) have noted a correlation between the severity of carotid atherosclerotic lesion and basal neutrophil count. In their study, the number of macrophages was increased and neutrophil count was higher in the presence of rupture-prone atherosclerotic plaques that are characterized by low collagen content and smooth muscle cells. Previous studies have established that the activated neutrophil count in the circulation is higher in patients with coronary and carotid atherosclerotic plaques, and neutrophils play crucial roles in the development of atherosclerotic plaques as well as rupture of a pre-existing plaque.23)
Lymphopenia is the most common inflammatory marker that develops in response to increased corticosteroid level secondary to stress24) and it occurs due to the increased apoptosis of lymphocytes during inflammatory process.25) Lymphopenia has been associated with mortality in patients who have experienced ST-elevation myocardial infarction.26) NLR is considered to be a marker that simultaneously shows the negative effects of neutrophil elevation as an indicator of acute inflammation and lymphocyte depletion as an indicator of physiological stress.11) NLR can be easily calculated based on the white blood cell count, and it is also used as a marker of mortality and prognosis in patients who have experienced an ischemic stroke.10) Since NLR reflects both the neutrophil and lymphocyte counts, we believe that it is a more reliable marker than neutrophil or lymphocyte count alone to assess the inflammatory status of the carotid plaques that cause intermediate stenosis.
Our study group consisted of asymptomatic patients who had intermediate stenosis of the carotid artery. This patient group is currently being followed up by medical means in the light of available information. It is difficult to predict which of these patients will become symptomatic during follow-up. The nature of an atherosclerotic plaque is an important determinant of its symptomatic characteristics. Studies performed on coronary artery plaques showed that rupture-prone plaques are more active in terms of inflammation.27)28) Non-calcified carotid plaques are more unstable than calcified plaques, and therefore, they are associated with a higher risk of rupture, thromboembolism and stroke.16) van Lammeren et al.16) showed that patients with truly asymptomatic carotid atherosclerotic plaques have relatively more stable plaque characteristics with a higher plaque smooth muscle cell content, a higher proportion of heavily calcified plaques, and less frequent intraplaque haemorrhages compared with patients who had ipsilateral cerebrovascular events.
In the present study, NLR was higher in the non-calcified plaque group compared to the calcified plaque group; confirming that non-calcified carotid artery plaques are more active in terms of inflammation. Based on this finding, it may be argued that among the non-calcified plaques that cause asymptomatic intermediate carotid artery stenosis, those that are associated with increased NLR (NLR cut-off value of >2.54 was found in our study) may reflect a higher risk of stroke and such cases should be closely monitored.
Hemoglobin level was significantly lower in the non-calcified plaque group compared to the calcified plaque group. The reason for such a difference between the two groups with respect to hemoglobin levels is not clear. We believe this may be due to the higher rate of ASA use in the non-calcified plaque group and the associated non-clinical gastrointestinal bleeding events.
NLR can be affected by coexisting comorbidities and concomitant medical therapies, particularly by statins. In the present study, blood samples were obtained from all the analyzed patients after their comorbidities had been stabilized and they had regularly used medical therapy for at least 2 months. Mean values of the parameters measured in the blood samples drawn at two different time points were analyzed. Arrangements have been made to ensure that the hematological markers that are evaluated as the endpoints of this study reflect the actual values.
An important factor that distinguishes the present study from previous studies is that in this study, carotid stenosis and plaques were assessed by both CDUS and CTA. CTA is a more convenient method than magnetic resonance angiography and Doppler ultrasonography for assessing the severity of carotid artery stenosis.29) Occasionally, the degree of stenosis as demonstrated by CDUS and by CTA may be different. Assessing carotid plaques by both these methods reduces the margin of error. CTA is a particularly reliable method to evaluate the nature of a plaque and extent of plaque calcification in carotid arteries.30)
One of the limitations of this study is the lack of comparison between neutrophil and lymphocyte counts and the levels of other inflammatory markers. All of the comorbidities and environmental factors that might have affected the inflammatory markers were not taken into account. Also, our findings may not reflect the outcomes in female patients since the majority of the study cohort was male. Additional limitations of this study may be listed as it being a single-center study and its relatively small sample size.
Figures and Tables
Fig. 2
Comparison of NLR between the two groups demonstrated that it was statistically significantly higher in the non-calcified plaque group (p<0.001). NLR: neutrophil/lymphocyte ratio.

Fig. 3
In ROC curve analysis, NLR cut-off value of >2.54 was found for calcified-non calcified plaque discrimination among carotid arteries plaques which caused intermediate obstruction. NLR: neutrophil/lymphocyte ratio, AUC: area under curre.

Table 1
Comparison of the demographical, clinical and biochemical characteristics between the groups

Table 2
Comparison of the hematological parameters between the groups

Table 3
Independent predictors of non-calcified carotid plaque in multivariate logistic regression analysis
References
1. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008; 39:2396–2399.
2. Cybulsky MI, Gimbrone MA Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991; 251:788–791.
3. Kolodgie FD, Burke AP, Farb A, et al. The thin-cap fibroatheroma: A type of vulnerable plaque: The major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001; 16:285–292.
4. Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010; 122:1837–1845.
5. Larionov S, Dedeck O, Birkenmeier G, Thal DR. Expression of alpha2-macroglobulin, neutrophil elastase, and interleukin-1alpha differs in early-stage and late-stage atherosclerotic lesions in the arteries of the circle of Willis. Acta Neuropathol. 2007; 113:33–43.
6. Leclercq A, Houard X, Philippe M, et al. Involvement of intraplaque hemorrhage in atherothrombosis evolution via neutrophil protease enrichment. J Leukoc Biol. 2007; 82:1420–1429.
7. Thomson SP, Gibbons RJ, Smars PA, et al. Incremental value of the leukocyte differential and the rapid creatine kinase-MB isoenzyme or the early diagnosis of myocardial infarction. Ann Intern Med. 1995; 122:335–341.
8. Dragu R, Khoury S, Zuckerman R, et al. Predictive value of white blood cell subtypes for long-term outcome following myocardial inrafction. Atherosclerosis. 2008; 196:405–412.
9. Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008; 102:653–657.
10. Gökhan S, Ozhasenekler A, Mansur Durgun H, Akil E, Ustündag M, Orak M. Neutrophil lymphocyte ratios in stroke subtypes and transient ischemic attack. Eur Rev Med Pharmacol Sci. 2013; 17:653–657.
11. Gibson PH, Cuthbertson BH, Croal BL, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2010; 105:186–191.
12. Ahmadi N, Nabavi V, Hajsadeghi F, et al. Mortality incidence of patients with non-obstructive coronary artery disease diagnosed by computed tomography angiography. Am J Cardiol. 2011; 107:10–16.
13. van Werkhoven JM, Schuijf JD, Gaemperli O, et al. Incremental prognostic value of multi-slice computed tomography coronary angiography over coronary artery calcium scoring in patients with suspected coronary artery disease. Eur Heart J. 2009; 30:2622–2629.
14. Hadamitzky M, Freissmuth B, Meyer T, et al. Prognostic value of coronary computed tomographic angiography for prediction of cardiac events in patients with suspected coronary artery disease. JACC Cardiovasc Imaging. 2009; 2:404–411.
15. Hunt JL, Fairman R, Mitchell ME, et al. Bone formation in carotid plaques: a clinicopathological study. Stroke. 2002; 33:1214–1219.
16. van Lammeren GW, den Hartog AG, Pasterkamp G, et al. Asymptomatic carotid artery stenosis: identification of subgroups with different underlying plaque characteristics. Eur J Vasc Endovasc Surg. 2012; 43:632–636.
17. Liapis CD, Bell PR, Mikhailidis D, et al. ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg. 2009; 37:4 Suppl. 1–19.
18. Brott TG, Halperin JL, Abbara S, et al. Guideline on the management of patients with extracranial carotid and vertebral artery disease. J Am Coll Cardiol. 2011; 57:1002–1044.
19. Wang PQ, Wang Y, Zhang GB, Zhou PY, Liu JZ, Wang SA. Study on the carotid atherosclerotic plaque of patients suffering from ischemic cerebrovascular disease by 64 slices CT. Eur Rev Med Pharmacol Sci. 2015; 19:3480–3485.
20. Mathers CD, Boerma T, Ma Fat D. Global and regional causes of death. Br Med Bull. 2009; 92:7–32.
21. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012; 32:2045–2051.
22. Nasr N, Ruidavets JB, Arnal JF, Sie P, Larrue V. Association of neutrophil count with microembolization in patients with symptomatic carotid artery stenosis. Atherosclerosis. 2009; 207:519–523.
23. Ionita MG, van den Borne P, Catanzariti LM, et al. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2010; 30:1842–1848.
24. Onsrud M, Thorsby E. Influence of in vivo hydrocortisone on some humab blood lymphocyte subpopulations. Effect on natural killer cell activity. Scand J Immunol. 1981; 13:573–579.
25. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003; 348:138–150.
26. Pellizzon GG, Dixon SR, Gregg W, et al. Relation of admission white blood cell count to long-term outcomes after primary coronary angioplasty for acute myocardial infarction (The Stent PAMI Trial). Am J Cardiol. 2003; 91:729–731.
27. Naruko T, Ueda M, Haze K, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002; 106:2894–2900.
28. Tavora FR, Ripple M, Li L, Burke AP. Monocytes and neutrophils expressing myeloperoxidase occur in fibrous caps and thrombi in unstable coronary plaques. BMC Cardiovasc Disord. 2009; 9:27.
29. Bozzao A, Floris R, Pocek M, Fasoli F, Garaci FG, Simonetti G. Non-invasive assessment of epiaortic vessels. Comparison of magnetic resonance angiography with gadolinium, spiral computerized tomography angiography, and digital angiograph. Radiol Med. 2001; 101:48–53.
30. Hingwala D, Kesavadas C, Sylaja PN, Thomas B, Kapilamoorthy TR. Multimodality imaging of carotid atherosclerotic plaque: Going beyond stenosis. Indian J Radiol Imaging. 2013; 23:26–34.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download