Introduction
Celiac Disease (CD) is a chronic small bowel immune-mediated enteropathy precipitated by exposure to dietary gluten in genetically predisposed individuals.1) CD affects approximately 1% of the European population.2) Patients are intolerant to dietary gluten and recurrent exposure causes chronic inflammation in the small intestines, villous atrophy and crypt hyperplasia.3) Genetic, environmental and autoimmune factors play a role in its etiology and CD is associated with other autoimmune disorders such as autoimmune thyroiditis, type 1 diabetes mellitus, collagen tissue diseases and giant cell myocarditis4)5)6)
Case
A 41-year-old male patient was admitted to the emergency department with ongoing chest pain. The patient had no risk factors for atherosclerosis and hemodynamic parameters were stable upon admission. There was no history of trauma or collagen tissue disease and he was not currently taking any medication. He was diagnosed with CD 12 years earlier and had not been on a gluten free diet (GFD) for a year. An electrocardiogram found nonspecific ST-T changes. However, the troponin I level was 22 ng/mL, which is well above the reference range (0.02-0.06 ng/mL). The patient was therefore admitted to the coronary intensive care unit with a primary diagnosis of non-ST segment elevation myocardial infarction. An echocardiogram revealed severely hypokinetic inferior, posterior and lateral walls of the left ventricle, with an ejection fraction of 35% (Normal >52%) in the absence of a primary valve disorder. Bloodwork hemoglobin was 9.3 g/dL, white blood cell count was 2.25×104/mL and platelet count was 4.41×105/mL. Lipid levels as well as liver and kidney function tests were normal, but erythrocyte sedimentation rate was 19 mm/hour (0-15 mm/h) and C-reactive protein (CRP) was 12.5 mg/dL (0-6 mg/dL).
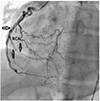
A coronary angiography (CAG) was performed and diffuse dissections within the left anterior descending artery (LAD) and the right coronary artery (RCA) were observed with total occlusion of second obtuse marginal artery (Figs. 1 and 2). Severely reduced left ventricular dysfunction was detected on ventriculography. Because the diffuse dissections involved many vessels, coronary artery bypass grafting (CABG) was selected as the best treatment. The homocysteine level was measured and found to be elevated at 20 µmol/L (Normal 4.3-11.4 µmol/L).
Anti-nuclear antibody, anti ds-DNA and rheumatoid factor levels were within normal limits. No mutations were found in factor-5-Leiden, prothrombin 20210 and antithrombin III. The patient successfully underwent a coronary artery bypass graft surgery and is currently being monitored by a gastroenterologist. He is also back on a gluten free diet.
Discussion
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome. SCAD generally occurs in people between the ages of 30-50 and tends to occur more in women that do not have risk factors for atherosclerosis. SCAD often occurs in the peripartum period or due to chest trauma, intravenous drug abuse, cocaine usage and severe hypertension, as well as collagen tissue diseases, autoimmune thyroiditis and inflammatory bowel diseases.
The angiographic incidence of SCAD was reported to be 0.28-1.1%. SCAD is generally seen in one vessel and women are more likely to have LAD dissections while men tend to have more RCA dissections.7) One of the supposed mechanisms of SCAD pathophysiology is eosinophilic infiltration of the vessel wall followed by the release of lytic enzymes, which causes dissection.
Dissection causes ischemic events by decreasing true lumen blood flow as the vessel wall lamina cleaves due to intramural hemorrhage and/or hematoma into the false lumen.
Multivessel SCAD is a very rare disorder and its etiology is not yet completely understood. The case discussed in this paper demonstrated a relationship between multivessel SCAD and an elevated homocysteine level.8) In our case, the patient with CD had no risk factors for artherosclerosis or any other related causes. Therefore, we postulate that systemic inflammation due to CD and an elevated homocysteine level may have played a role in the development of multivessel SCAD in this case.
Celiac disease is a common disease affecting mainly the small intestine, though it can affect other organs as well, and presents a wide range of symptoms with varying levels of severity. Patients with CD may experience malabsorption that manifests as diarrhea, steatorrhea, weight loss and nutritional deficiencies. CD is currently diagnosed using Immunoglobulin A (IgA) anti-tissue transglutaminase (tTG) antibody (IgAtTG) followed by confirmatory biopsies of the small intestine to search for histopathological findings compatible with CD.9)10) The risk of thromboembolic complications are increased in inflammatory bowel disease due to higher levels of proinflammatory cytokines,11) though there may be conflicting results in CD patients. Ludvigsson et al.12) demonstrated a positive correlation between CD and myocardial infarction, angina pectoris, congestive heart failure, intracranial bleeding and ischemic stroke. De Marchi et al.13) showed a relationship with increased carotid intima-media thickness (CIMT) and decreased flow-mediated dilatation (FMD) in addition to elevated CRP and homocysteine levels in recently diagnosed young CD patients. De Marchi et al.13) also showed that GFD improved CIMT, FMD and CRP levels but not homocysteine levels. This findings support the theory that chronic inflammation in CD patients increases the risk of premature atherosclerosis.
The symptoms of malabsorption are alleviated with GFD. However, vitamin B6, B12 and folate deficiencies might still be observed in patients with malabsorption. This may cause hyperhomocysteinemia, which may increase the risk of vascular diseases. Hyperhomocysteinemia is a possible risk factor of coronary atherosclerosis in CD patients. Hyperhomocysteinemia may cause endothelial dysfunction, activation of tissue-type plasminogen activator and factor V, increased platelet aggregation and inhibition of protein C, all of which can result in plaque rupture and vascular damage.14) There are studies demonstrating that supplementation of vitamins B6 and B12 does not reduce homocysteine levels.15)
The diagnosis of SCAD on CAG is made with the visualization of a radiolucent intimal flap and double lumen.16) Treatment is determined by clinical findings in the individual patient and the extent of dissection. Hemodynamically stable patients without persistent ischemia and with one dissected vessel are likely to be medically managed. Some cases are also self-limiting and resolve on their own.17) Percutaneous coronary intervention is the best treatment in cases where the dissection is short segmented and involves only one vessel. However, the procedure has a lower success rate and higher complication rate compared to percutaneous coronary interventions in patients without dissection. Surgery is recommended cases with left main artery dissection or multivessel dissections such as in this case.18)
In conclusion, CD is a rare cause of myocardial infarction. Chronic inflammation and hyperhomocysteinemia may increase cardiovascular risk in CD patients. SCAD development appears to be a rare extraintestinal manifestation of CD.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download