Abstract
Background and Objectives
Coronary artery ectasia (CAE) is an angiographic finding characterized by dilation of an arterial segment with a diameter at least 1.5 times that of its adjacent normal coronary artery. Fragmented QRS (fQRS) complexes are electrocardiographic signals which reflect altered ventricular conduction around regions of a myocardial scar and/or ischaemia. In the present study, we aimed to evaluate the presence of fQRS in patients with CAE.
Subjects and Methods
The study population included 100 patients with isolated CAE without coronary artery disease (CAD) and 80 angiographically normal controls. fQRS was defined as the presence of an additional R wave or notching of R or S wave or the presence of fragmentation in two contiguous leads corresponding to a major coronary artery territory.
Results
The two groups were similar in terms of age, sex, hypertension, dyslipidemia, and family history of CAD. The presence of fQRS was significantly (p<0.05) higher in the CAE group than that in the normal coronary artery group (29% vs. 6.2%, p=0.008). Isolated CAE were detected most commonly in the right coronary artery (61%), followed by left anterior descending artery (52%), left circumflex artery (36%), and left main artery (9%). Multivariate stepwise logistic regression analysis showed that CAE {odds ratio (OR) 1.412; 95% confidence interval (CI) 1.085-1.541; p=0.003} and diabetes (OR 1.310; 95% CI 1.025-1.482; p=0.041) were independently associated with fQRS.
Conclusion
The presence of fragmented QRS associated with increased risk for arrhythmias and cardiovascular mortality was significantly higher in patients with CAE than in patient with normal coronary artery. Further studies are needed to determine whether the presence of fragmented QRS is a possible new risk factor for patients with CAE.
Coronary artery ectasia (CAE), an aberration of the coronary anatomy, has been characterized as dilation of an arterial segment with a diameter at least 1.5 times that of its adjacent normal coronary artery.1)2) Underlying etiological causes of CAE include atherosclerosis (50%), congenital origins (20-30%), inflammatory and connective tissue diseases (20-30%).3)4) CAE may result in slowed blood flow, coronary vasospasm, dissection, and thrombus formation, leading to increased risk of cardiac morbidity and mortality.5)6)7)
Fragmented QRS (fQRS) complexes are novel electrocardiographic signals which reflect altered ventricular conduction around regions of a myocardial scar. fQRS is defined as the presence of slurred QRS complexes with various RSR' patterns without typical bundle branch block in two contiguous leads corresponding to a major coronary artery territory.8) The presence of fQRS complexes in a routine 12-lead electrocardiography (ECG) is a marker for abnormal cardiac depolarisation. It has been demonstrated that the presence of fQRS in patients with coronary artery disease (CAD) has been associated with regional myocardial damage, increased adverse cardiac events, and decreased event-free survival.9)10)11) Hence, fQRS may be a reliable indicator of past myocardial ischaemia in the absence of Q waves. In addition, fQRS has been associated with arrhythmic events in patients with Brugada syndrome12) and non-ischaemic cardiomyopathy.13)
To the best of our knowledge, fQRS in patients with CAE was not reported previously. It was unclear whether CAE was associated with fQRS. The presence of fQRS on ECG may be an indicator of myocardial damage in patients with CAE. Therefore, the purpose of this study was to evaluate the presence of fQRS in patients with CAE.
The study population consisted of 180 patients including 100 patients with isolated CAE without CAD and 80 angiographically normal controls, who underwent coronary angiography in our center.
Patients with a history of cardiomyopathy and myocardial infarction (MI), left ventricular hypertrophy (LVH), pathological Q wave on ECG, typical left bundle block or right bundle block, incomplete right bundle block, or paced rhythm on ECG were excluded from this study. Echocardiographic examinations were performed in all subjects. LVH was excluded by using echocardiography. MI and necrosis were evaluated based on history, ECG, echocardiography, and left ventriculography. Patients who were taking medications that could affect the ECG such as antiarrhythmics, beta-blockers, and calcium antagonists were also excluded from the study.
Coronary angiography was performed using the Judkins technique through femoral artery access. Coronary angiograms were analyzed by two experienced interventional cardiologists without knowledge of the ECG, laboratory measurements, or clinical status of the participant. CAE was defined as the segmental or diffuse dilation of the coronary arteries with a diameter >1.5 times of its adjacent segments of the same artery or of different arteries.3) Normal coronary artery was defined as coronary arteries without ectasia or stenosis on the basis of coronary angiography. The classification of CAE was based on the recommendation of Markis et al.14) and graded as the following: 1) type 1, diffuse ectasia of two coronary arteries; 2) type 2, diffuse ectasia in one coronary artery and localized ectasia in another coronary artery; 3) type 3, diffuse ectasia of one coronary artery; 4) type 4, localized or segmental (focal) ectasia of only one coronary artery. Based on the classification methods by Markis et al.,14) types 1, 2, and 3 are classified as diffuse ectasia whereas type 4 is classified as focal ectasia.
A 12-lead surface ECG was obtained from all patients in supine position. We used 12 lead ECG machine (MAC 1200, General electric, Milwaukee, WI, USA) with the following setting: filter range 0.16-100 Hz, AC filter 60 Hz, paper speed 25 mm/s and 10 mm/mV. Fragmentation was defined as the presence of various RSR' patterns with different morphologies of QRS complexes. Various RSR' patterns included additional R wave (R'), notching of the R wave or the S wave, or the presence of >1 R' (fragmentation) without a typical bundle branch block in 2 contiguous leads corresponding to a major lead set for major coronary artery territory. Any QRS morphology with a QRS duration >120 ms, including bundle branch block or intraventricular conduction delay, was excluded. Analysis of the standard 12-lead ECG was performed without using any magnification. Fragmentations were considered to be present if a visually identifiable signal was demonstrated in all complexes of a particular lead. For statistical analysis, fQRS was defined to be present in ≥2 contiguous anterior leads, lateral leads, or inferior leads. QRS duration was determined by the longest QRS in any lead. All ECG were assessed by a single operator who had no knowledge of the angiographycal, clinical, or laboratory characteristics of the patients.
All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Variables were investigated using visual (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov test) to determine whether their distributions were normal. Descriptive analyses were presented as mean±standard deviation. Categorical variables were expressed as percentages. All continuous variables were given as mean±SD; categorical variables were defined as percentages. Categorical data was compared with the χ2 test, Yates Continuity Correction and Fisher's Exact test. Mean values of continuous variables were compared between groups using the Student t-test or Mann-Whitney U test. Data were analysed to find out independent predictors of CAE with multivariate logistic regression analysis. P values of less than 0.05 were considered significant.
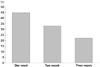
Clinical and laboratory findings of the subjects are shown in Table 1. The two groups were not significantly different from each other in terms of age, sex, hypertension, dyslipidemia, and family history of CAD (p>0.05). In addtion, there was no significant difference between the two groups in terms of body mass index and ejection fraction (p>0.05). However, diabetes mellitus and smoking was significantly (p<0.05) more common in the CAE group than those in the normal coronary artery group (p=0.041, p=0.001). In addition, the presence of fQRS was significantly (p<0.05) higher in the CAE group than that in the normal coronary artery group (29% vs. 6.2%, p=0.008). Our results also revealed that isolated CAE were detected most commonly in the right coronary artery (61%), followed by left anterior descending artery (52%), left circumflex artery (36%), and left main artery (9%) (Table 2). Additionally, isolated CAE were most frequently involved in one vessel (45%) and in three vessels (33%), but less frequently in two vessels (22%) (Fig. 1). Based on the classification by Markis et al.,14) the incidence of type I, II, III and IV lesions was 49%, 9%, 36%, and 6%, respectively.
Electrocardiography analysis of 29 patients with CAE and fQRS are shown in Table 3. All patients with ectasia in the right coronary artery had fQRS in the inferior leads (DII, DIII, aVF) except one patient who had fQRS in both the inferior and the anterior leads (V 1-4). All patients with coronary ectasia in the left anterior descending artery had fQRS in anterior leads except two patients who had fQRS in both anterior and lateral leads (V 4-6, DI, aVL).
A total of 15 of 29 patients with CAE and fQRS had Holter analysis in their past data records (Table 4). A total of 32 of 71 patients with CAE without fQRS also had Holter analysis in their past data records (Table 4). Patients with fQRS appeared to have more extrasystoles than patients without fQRS. However, such difference was not statistically significant (p=0.059) (Table 4). Multivariate stepwise logistic regression analysis showed that CAE {odds ratio (OR) 1.212; 95% confidence interval (CI) 1.085-1.541; p=0.003} and diabetes mellitus (OR 1.110; 95% CI 1.025-1.482; p=0.041) were independently associated with fQRS (Table 5).
This study revealed that the presence of fQRS was significantly higher in the CAE group than that in the normal coronary artery group, suggesting that the presence of fQRS might be an indicator for CAE. Isolated CAE is a common finding in coronary disorder in the era of coronary angiography. The clinical features and mechanisms invovled in this unique coronary disorder are unclear. Some inflammatory markers such as C-reactive protein, interleukin 6, tumor necrosis factor a, matrix metalloproteinase, hypertension and smoking have been reported to be associated with CAE.15)16)17)18) However, some atherosclerotic risk factors such as advanced age and diabetes have been reproted to be inversely associated with CAE.18)19) CAE is reported in 1.5% to 5% of patients used in coronary angiographic studies.20) Angina pectoris can be seen in patients with CAE without CAD. In addition, some studies14)15)16)17)18)19)20)21) have shown that the frequency of acute coronary events via vasospasm, dissection, or thrombus is higher in patients with isolated CAE than in patients with normal coronary angiograms. Moreover, it has been reported that 29% to 39% of patients with isolated ectasia have a history of previous MI or angina pectoris and that patients with CAE have an increased risk of mortality equivalent to patients with CAD.21)
Fragmentation of QRS complex is an easy and non-invasive electrocardiographic parameter associated with inhomogeneous activation of the ventricles and myocardial conduction delays due to myocardial scar and/or ischaemia, which could predict arrhythmic events as well as death. QRS fragmentation analyzed from surface ECG has appeared as a new risk marker for many diseases such as CAD, nonischemic cardiomyopathy (hypertrophic, dilated, Chagas' disease, arrhythmogenic right ventricular cardiomyopathy, fallot, and sarcoidosis), and ion cannel diseases including Brugada syndrome and long QT syndrome.22)23)24)25)26)27) Das et al.,22) the first ones who described the presence of fQRS in patients with CAD, have demonstrated its good sensitivity and specificity for the prediction of myocardial scar in patients with poor prognosis associated with this ECG presentation. The underlying mechanisms of fragmentation have been determiend by autopsy studies of patients with MI. Studies have shown that the presence of fQRS is associated with significant myocardial necrosis alternating with viable myocardial tissue and interspersed in abundant fibrous tissue.11)28)
Individual case reports have shown that isolated CAE alone may be a cause of silent myocardial ischemia and infarction.4)29) It was reported that coronary flow reserve was significantly reduced in patients with CAE compared to matched control subjects.7) Akyürek et al.7) suggested that microvascular dysfunction might be the underlying cause of exercise-induced myocardial ischemia. Taken together, these results suggest that microvascular dysfunction and/or ischemia might be a reason behind the fragmanted QRS in patients with CAE. It has been shown that CAE could be the cause of transient myocardial hypoperfusion in patients with angina and normal coronary arteries.21) Whether CAE is associated with fQRS is unknown. In our study, the frequency of fQRS complexes was significantly higher in patients with CAE compared to that in patients of the normal coronary artery. A poor myocardial perfusion might be the cause of ischaemia and the occurrence of micro infarctions. Thus, CAE might be responsible for depolarisation abnormalities in these patients.
Fragmented QRS, an indicator for increased risk of arrhythmias and cardiovascular mortality, was found to be significantly higher in patients with CAE. The presence of fQRS on ECG may be an indicator of myocardial damage in patients with CAE. Further studies are needed to establish its significance as a possible new risk factor in patients with CAE.
Our study has some limitations. Firstly, our results are based on a relatively small sample size. Therefore, these findings must be confirmed by further large-scale prospective studies. Secondly, although a significant association between CAE and fQRS was observed, we could not establish the exact underlying mechanisms responsible for this association. Thirdly, relation of CAE with myocardial scar or ischemia can be accurately quantified by using magnetic resonance imaging (MRI) or computed tomography (CT) imaging. However, none of the patients in our study underwent MRI or CT to show myocardial scar.
Figures and Tables
Table 1
Demographic and clinical features of study subjects
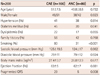
Table 2
Anatomic localizations of coronary artery ectasia

| Localizations of CAE (n=100) | % |
|---|---|
| Left main coronary artery | 9 |
| Left anterior descending artery | 52 |
| Left circumflex artery | 36 |
| Right coronary artery | 61 |
Table 3
Relation of anatomic localization of CAE and presented leads of fQRS on ECG

Table 4
Twenty-four hours ECG holter monitoring results of patients

References
1. Satran A, Bart BA, Henry CR, et al. Increased prevalence of coronary artery aneurysms among cocaine users. Circulation. 2005; 111:2424–2429.
2. Li JJ, He JG, Nan JL, He ZX, Zhu CG, Li J. Is systemic inflammation responsible for coronary artery ectasia? Int J Cardiol. 2008; 130:e69–e70.
3. Falsetti HL, Carrol RJ. Coronary artery aneurysm. A review of the literature with a report of 11 new cases. Chest. 1976; 69:630–636.
4. Befeler B, Aranda MJ, Embi A, Mullin FL, El-Sherif N, Lazzara R. Coronary artery aneurysms: study of the etiology, clinical course and effect on left ventricular function and prognosis. Am J Med. 1977; 62:597–607.
5. Krüger D, Stierle U, Herrmann G, Simon R, Sheikhzadeh A. Exercise-induced myocardial ischemia in isolated coronary artery ectasias and aneurysms ("dilated coronopathy"). J Am Coll Cardiol. 1999; 34:1461–1470.
6. Mattern AL, Baker WP, McHale JJ, Lee DE. Congenital coronary aneurysms with angina pectoris and myocardial infarction treated with saphenous vein bypass graft. Am J Cardiol. 1972; 30:906–909.
7. Akyürek O, Berkalp B, Sayin T, Kumbasar D, Kervancioğlu C, Oral D. Altered coronary flow properties in diffuse coronary artery ectasia. Am Heart J. 2003; 145:66–72.
8. Tigen K, Karaahmet T, Gurel E, et al. The utility of fragmented QRS complexes to predict significant intraventricular dyssynchrony in non-ischemic dilated cardiomyopathy patients with a narrow QRS interval. Can J Cardiol. 2009; 25:517–522.
9. Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006; 113:2495–2501.
10. Michael MA, El Masry H, Khan BR, Das MK. Electrocardiographic signs of remote myocardial infarction. Prog Cardiovasc Dis. 2007; 50:198–208.
11. Das MK, Saha C, El Masry H, et al. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007; 4:1385–1392.
12. Take Y, Morita H, Toh N, et al. Identification of high-risk syncope related to ventricular fibrillation in patients with Brugada syndrome. Heart Rhythm. 2012; 9:752–759.
13. Das MK, Maskoun W, Shen C, et al. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010; 7:74–80.
14. Markis JE, Joffe CD, Cohn PF, Feen DJ, Herman MV, Gorlin R. Clinical significance of coronary arterial ectasia. Am J Cardiol. 1976; 37:217–222.
15. Aydin M, Tekin IO, Dogan SM, et al. The levels of tumor necrosis factor-alpha and interleukin-6 in patients with isolated coronary artery ectasia. Mediators Inflamm. 2009; 2009:106145.
16. Dogan A, Tuzun N, Turker Y, Akcay S, Kaya S, Ozaydin M. Matrix metalloproteinases and inflammatory markers in coronary artery ectasia: their relationship to severity of coronary artery ectasia. Coron Artery Dis. 2008; 19:559–563.
17. Finkelstein A, Michowitz Y, Abashidze A, Miller H, Keren G, George J. Temporal association between circulating proteolytic, inflammatory and neurohormonal markers in patients with coronary ectasia. Atherosclerosis. 2005; 179:353–359.
18. Aboeata AS, Sontineni SP, Alla VM, Esterbrooks DJ. Coronary artery ectasia: current concepts and interventions. Front Biosci (Elite Ed). 2012; 4:300–310.
19. Huang QJ, Liu J, Chen MH, Li JJ. Relation of diabetes to coronary artery ectasia: a meta-analysis study. Anadolu Kardiyol Derg. 2014; 14:322–327.
20. Boles U, Eriksson P, Zhao Y, Henein MY. Coronary artery ectasia: remains a clinical dilemma. Coron Artery Dis. 2010; 21:318–320.
21. Farto e Abreu P, Mesquita A, Silva JA, Seabra-Gomes R. [Coronary artery ectasia: clinical and angiographic characteristics and prognosis]. Rev Port Cardiol. 1993; 12:305–310.
22. Priori SG, Gasparini M, Napolitano C, et al. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012; 59:37–45.
23. Das MK, Suradi H, Maskoun W, et al. Fragmented wide QRS on a 12-lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008; 1:258–268.
24. Femenia F, Arce M, Arrieta M, Baranchuk A. Surface fragmented QRS in a patient with hypertrophic cardiomyopathy and malignant arrhythmias: is there an association? J Cardiovasc Dis Res. 2012; 3:32–35.
25. Sha J, Zhang S, Tang M, Chen K, Zhao X, Wang F. Fragmented QRS is associated with all-cause mortality and ventricular arrhythmias in patient with idiopathic dilated cardiomyopathy. Ann Noninvasive Electrocardiol. 2011; 16:270–275.
26. Baranchuk A, Miranda R, Femenia F. FECHA Investigators. Chagas' cardiomyopathy and Fragmented QRS. Re: QRS fragmentation as a marker of arrhythmias in coronary artery disease, in cardiomyopathies and ion channel diseases. Int J Cardiol. 2012; 160:151–152.
27. Haraoka K, Morita H, Saito Y, et al. Fragmented QRS is associated with torsades de pointes in patients with acquired long QT syndrome. Heart Rhythm. 2010; 7:1808–1814.
28. Das MK, Zipes DP. Fragmented QRS: a predictor of mortality and sudden cardiac death. Heart Rhythm. 2009; 6:3 Suppl. S8–S14.
29. Nagata K, Kawasaki T, Okamoto A, et al. Effectiveness of an antiplatelet agent for coronary artery ectasia associated with silent myocardial ischemia. Jpn Heart J. 2001; 42:249–254.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download