Abstract
Background and Objectives
The aim of this study was to investigate the impact of treatment with oral trimetazidine (TMZ) applied before and after percutaneous coronary interventions (PCI) on short-term left ventricular functions and plasma brain natriuretic peptide (BNP) levels in patients with non-ST segment elevation myocardial infarction (NSTEMI) undergoing PCI.
Subjects and Methods
The study included 45 patients who were undergoing PCI with the diagnosis of NSTEMI. The patients were randomized into two groups. The first group (n=22) of the patients hospitalized with the diagnosis of NSTEMI was given conventional therapy plus 60 mg TMZ just prior to PCI. Treatment with TMZ was continued for one month after the procedure. TMZ treatment was not given to the second group (n=23). Echocardiography images were recorded and plasma BNP levels were measured just prior to the PCI and on the 1st and 30th days after PCI.
Results
The myocardial performance index (MPI) was greater in the second group (p=0.02). In the comparison of BNP levels, they significantly decreased in both of the groups during the 30-day follow-up period (29.0±8 and 50.6±33, p<0.01 respectively). However, decreasing of BNP levels was higher in the group administered with TMZ. The decrease of left ventriclular end-diastolic volume was observed in all groups at 30 days after intervention, but was higher in the group administered with TMZ (p=0.01).
Despite rapid advancements in the therapeutic field of cardiovascular diseases, acute coronary syndromes (ACSs) are still the leading cause of morbidity and mortality worldwide. Increased numbers of coronary intensive care units, reperfusion techniques for percutaneous coronary interventions (PCIs), and advanced medical therapies, including several pharmacological agents, have resulted in a significantly prolonged life expectancy. However, despite intensive therapies with hemodynamically effective agents, many patients with ischemic disease do not recover fully and remain at high risk for undesired further events. Hence, attempts to develop novel therapies are currently ongoing.
Trimetazidine (TMZ) has an anti-ischemic effect through the selective inhibition of long-chain 3-ketoacyl-CoA thiolase and the direct stimulation of pyruvate dehydrogenase, which provides a shift in cardiac energy metabolism from fatty acid oxidation to glucose oxidation.1) As a result, TMZ preserves the necessary ATP level in cardiomyocytes, promotes a decrease in intracellular acidosis, and prevents intracellular calcium overload.2)3) It reduces myocardial injuries caused by free radicals and, therefore, modulates the inflammatory response. It can limit the necrotic area of the myocardium. Therefore, TMZ preserves the contractile function of myocardium, reducing ischemia and reperfusion damage following an ischemic attack.4) Elevated concentrations of brain natriuretic peptide (BNP) and the N-terminal portion of BNP prohormone, early and late after presentation with an ACS, are strongly associated with an increase in adverse cardiovascular events.5-7) The aim of this study was to investigate the impact of treatment with oral TMZ administered prior to and after PCI on short-term left ventricular (LV) functions and plasma BNP levels in patients with non-ST segment elevation myocardial infarction (NSTEMI) undergoing PCI.
In the present study, 45 patients with NSTEMI and critical coronary artery lesions, as shown in the selective coronary angiography, who underwent PCI were included. Patients were included due to symptoms of unstable coronary artery disease with objective signs of myocardial ischemia, such as electrocardiographic changes (ST-segment depression ≥0.1 mV or T-wave inversion ≥0.1 mV in 2 contiguous leads) or elevated biochemical markers of myocardial necrosis. Patients with severe left heart valve regurgitation, chronic renal insufficiency, chronic cor pulmonale, acute pulmonary embolism, severe heart valve disease, ST-segment elevation myocardial infarction, and hypertensive patients with LV hypertrophy were excluded. The study was approved by the local Ethics Committee. Informed consent forms were obtained from the patients.
Those patients who were scheduled for PCI according to coronary angiographic findings were randomly assigned into two groups. PCI was performed within 24 hours after admission. In Group 1, 22 patients underwent TMZ loading 60 mg before the procedure and conventional therapy plus TMZ 60 mg daily (3×20 mg) for one month following the procedure. In Group 2, 23 patients underwent PCI and followed up with conventional therapy alone.
All patients underwent standard coronary angiography via the femoral approach. Patients assigned to the early invasive strategy were scheduled to undergo an angiography within 4 to 48 hours after randomization, and percutaneous revascularization where appropriate, based on the coronary anatomy. The coronary stenosis severity in the vessel to be monitored during angioplasty was assessed visually and was defined as >90% in all patients. Immediately after the procedure, the angiographic control demonstrated a residual stenosis of <20% with Thrombolysis in Myocardial Infarction flow grade 3 in the dilated artery in all patients. Only patients with single-vessel disease were enrolled in the study. Routine care was taken before and after the procedure for all patients, including pretreatment with a loading dose of clopidogrel (300 mg initial oral bolus) the day prior to the procedure, followed by 75 mg/day for 1 month, in addition to aspirin medication (160 mg/day) and an intravenous bolus of unfractionated heparin (100 IU/kg) was administered at the beginning of the procedure.
All samples were collected by venipuncture into ethylenediaminetetraacetic acid tubes. The samples were analyzed within ten minutes using the Fluorescence Immunoassay technique and Biosite (CA, USA) using a BNP Triage Kit in the Biochemistry Lab, Emergency Unit. Laboratory parameters of BNP were assessed by a researcher who was blind to the clinical characteristics of the patients.
Transthoracic echocardiography was performed by one trained operator using the GE Vivid 7 system (GE Vingmed Ultrasound AS, Horten, Norway) with a 3.5-MHz transducer. All data were transferred to a workstation for further offline analysis (EchoPAC PC; GE Vingmed Ultrasound AS). Left ventricular ejection fraction (LVEF) was calculated using Simpson's formula from the measurement of end-diastolic and end-systolic volumes on apical 4-chamber views. Transmitral flow velocities (E and A) were obtained by a pulsed-wave Doppler in the apical four-chamber view. The ratio of E/A velocity and E-wave deceleration time were measured. Tissue Doppler imaging was used to measure mitral annular velocities. Early diastolic velocity (Em) and late diastolic velocity (Am) were obtained in the mitral septal annulus. Myocardial performance index (MPI) is a numeric value, obtained by using cardiac time intervals. This numeric value is defined as the sum of isovolumic contraction time and isovolumic relaxation time divided by ejection time.
Statistical analyses were performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Data are expressed as mean±SD or percentages for categorical variables. To compare parametric continuous variables, the independent Student t-test was used. For categorical variables, the χ2 test was used. P<0.05 was considered to be statistically significant.
A total of 45 patients were randomly divided into two groups: TMZ (22 patients) and control (23 patients). There were no significant differences in heart rate or systolic and diastolic blood pressure obtained during the echocardiographic examination. Both groups were comparable at the baseline with regard to their clinical characteristics, blood testing, echocardiographic evaluation, as well as the distribution of their concomitant treatment. Patients' characteristics at enrollment are reported in Table 1. All patients (n=45) completed the trial.
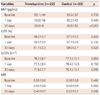
Left ventricular ejection fraction {TMZ group-58.2±3.7%, 59.1±3.9% and 61.7±2.3%; control group-57.5±2.5%, 57.7±2.6% and 59.0±2.7% at the baseline, at 1 day and at 30 days, respectively; p=not significant (NS)} (Fig. 1) as well as LV end-systolic volume (TMZ group-32.6±6.7, 31.2±5.9 and 27.5±4.0% cm3; control group-34.4±5.5 cm3, 33.6±5.6 cm3 and 32.6±6.2 cm3 at the baseline, at 1 day and at 30 days, respectively; p=NS) did not significantly change after 30 days of treatment.
Left ventricular end diastolic volume (LVEDV) TMZ group-78.7±9.7 cm3, 77.5±9.3 cm3 and 71.9±7.7 cm3; control group-77.1±13.1 cm3, 76.4±12.5 cm3 and 75.1±13.1 cm3 at the baseline, at 1 day and at 30 days, respectively (Fig. 1). While no difference was observed between LVEDV (p=0.05) levels, the decrease of LVEDV was observed in all groups at 30 days after intervention, but it was higher in the group administered TMZ treatment (p=0.01). BNP TMZ group-93.1±44 pg/mL, 73.0±16 pg/mL and 29.0±8 pg/mL; control group-85.0±37 pg/mL, 80.3±43 pg/mL and 50.6±33 pg/mL at the baseline, at 1 day and at 30 days, respectively (Fig. 1). BNP levels significantly decreased in both groups during the 30-day follow-up period (29.0±8 and 50.6±33, respectively). However, the decrease of BNP levels was higher in the group administered TMZ than the other group (p<0.01). MPI TMZ group-0.58±0.9, 0.55±0.8 and 0.48±0.7; control group-0.58±0.8, 0.56±0.9 and 0.52±0.1 at the baseline, at 1 day and at 30 days, respectively. Table 2 shows the differences between the baseline and the 30th day at LV ejection fraction, LVEDV, BNP, and MPI. MPI, which incorporates both systolic and diastolic time intervals in expressing global ventricular performance, was greater in the second group (p=0.02). Transmitral flow velocities (E and A), the ratio of E/A velocity and E-wave deceleration time, tissue Doppler imaging early diastolic velocity (Em), and late diastolic velocity (Am) between the two groups showed similar variations.
The present study demonstrated that short-term (30-day) TMZ treatment in patients with NSTEMI undergoing PCI reduces plasma levels of BNP, reduces LVEDV, and improves MPI.
Brain natriuretic peptide is synthesized in the ventricles as pre-pro BNP and transformed into pro-BNP. Thereafter, BNP is converted into biologically active BNP and biologically inactive N terminal-pro-BNP.8) BNP and NT-pro-BNP are released in response to ventricular myocardial contraction, indicating myocardial wall stress.9) It has been established in several clinical studies that increased BNP is related to ischemia, rather than myocardial necrosis in ACS. BNP is considered an important prognostic indicator for ACS.10-12) In our study, we found that plasma BNP levels, an indicator of myocardial stress, were lower with TMZ treatment initiated before PCI and continued for 30 days, compared to the controls.
Trimetazidine selectively inhibits fatty acid beta oxidation enzyme 3-ketoacyl-CoA dehydrogenase. This results in glucose oxidation, which is a more productive pathway for energy production, rather than fat oxidation.13) Shifting beta-oxidation to glucose oxidation, which requires lower oxygen demand, is beneficial for myocardial hypoperfusion, as ATP production per oxygen consumption unit (Mol) with glucose energy substrate is 12% higher compared to fatty acids. In addition, TMZ prevents calcium overload and cellular acidosis and, thereby, preserves cellular homeostasis by reducing the oxidation of fatty acids and reactivating the glucose pathway.14-16) Trimetazidine preserves myocardial contractile function, reducing ischemic damage and reperfusion damage following ischemia.17) Fragasso et al.18) investigated the effects of conventional therapy plus TMZ on New York Heart Association (NYHA) functional class, exercise tolerance, quality of life, and LV function in patients with heart failure, irrespective of the underlying etiology (i.e. ischemic or non-ischemic). The authors found that NYHA functional class was significantly improved with reduced end-systolic volume and significantly reduced LVEF in patients administered TMZ. Labrou et al.19) found that pre- and post-PCI TMZ therapy resulted in procedure-related myocardial damage with an improvement in the LV region, global wall movements, and LVEF at 1 and 3 months following PCI. In our study, we found no significant increase in LV ejection fraction in the TMZ group, compared to the controls. However, this study partially supports our results that a significant reduction in LVEDV and BNP levels was found in the TMZ group.
Mitral E and A velocities used for the assessment of LV diastolic functions, as shown in the Doppler echocardiography, were similar to the control group. No significant difference was observed between the groups in terms of Em and Am values of the Tissue Doppler parameters used for the assessment of diastolic functions. MPI is a numerical value obtained at cardiac time intervals. The Doppler index, irrespective of heart rate, is useful in the assessment of systolic and diastolic myocardial performance simultaneously. The MPI increases in patients with coronary artery disease accompanied by any degree of systolic dysfunction. In our study, we also found that MPI increased significantly at 30 days in patients receiving TMZ, compared to the control group. In addition to the MPI increase, the lack of improvement in EF may be explained by the relatively brief follow-up period.
In conclusion, TMZ treatment commenced prior to PCI and continued after PCI in patients with NSTEMI provided improvements in MPI, LVEDV, and a decrease in BNP levels. We concluded that the beneficial effects of PCI could be reinforced with a combination of PCI and TMZ treatment.
Our results should be applied only with caution to clinical situations in which acute ischemia is potentially involved, and further investigation is required. This study was based on a limited number of observations made in a small population of patients and a brief follow-up period, potentially diminishing the validity of the drawn statistical inference. The reason for the small study population was based on the need for acquiring homogeneity for the trial. Future studies are required for these findings to be applied to clinical practice.
Figures and Tables
Fig. 1
Changes in the left ventricular ejection fraction (LVEF), left ventricular end-disatolic volume (LVEDV), and plasma brain natriuretic peptide (BNP) levels during the follow-up period.

References
1. Szwed H, Sadowski Z, Elikowski W, et al. Combination treatment in stable effort angina using trimetazidine and metoprolol: results of a randomized, double-blind, multicentre study (TRIMPOL II). TRIMetazidine in POLand. Eur Heart J. 2001; 22:2267–2274.
2. Lopaschuk GD, Kozak R. Trimetazidine inhibits fatty acid oxidation in the heart. J Mol Cell Cardiol. 1998; 30:A112.
3. Aussedat J, Ray A, Kay L, Verdys M, Harpey C, Rossi A. Improvement of long-term preservation of isolated arrested rat heart: beneficial effect of the antiischemic agent trimetazidine. J Cardiovasc Pharmacol. 1993; 21:128–135.
4. American Heart Association. 1999 heart and stroke statistical update. Chicago: American Heart Association;1999.
5. Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000; 342:1163–1170.
6. Rosano GM, Vitale C, Fragasso G. Metabolic therapy for patients with diabetes mellitus and coronary artery disease. Am J Cardiol. 2006; 98:14J–18J.
7. Marazzi G, Wajngarten M, Vitale C, et al. Effect of free fatty acid inhibition on silent and symptomatic myocardial ischemia in diabetic patients with coronary artery disease. Int J Cardiol. 2007; 120:79–84.
8. Nakamura S, Naruse M, Naruse K, et al. Atrial natriuretic peptide and brain natriuretic peptide coexist in the secretory granules of human cardiac myocytes. Am J Hypertens. 1991; 4:909–912.
9. Yoshimura M, Yasue H, Okumura K, et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993; 87:464–469.
10. White HD, French JK. Use of brain natriuretic peptide levels for risk assessment in non-ST-elevation acute coronary syndromes. J Am Coll Cardiol. 2003; 42:1917–1920.
11. Jernberg T, Stridsberg M, Venge P, Lindahl B. N-terminal pro brain natriuretic peptide on admission for early risk stratification of patients with chest pain and no ST-segment elevation. J Am Coll Cardiol. 2002; 40:437–445.
12. Ruzyllo W, Szwed H, Sadowski Z, et al. Efficacy of trimetazidine in patients with recurrent angina: a subgroup analysis of the TRIMPOL II study. Curr Med Res Opin. 2004; 20:1447–1454.
13. Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000; 86:580–588.
14. Banach M. The role of trimetazidine in the treatment of heart diseases. Ponzan, Poland: Termedia Publishing House;2006. p. 52–56.
15. Blardi P, de Lalla A, Volpi L, Auteri A, Di Perri T. Increase of adenosine plasma levels after oral trimetazidine: a pharmacological preconditioning? Pharmacol Res. 2002; 45:69–72.
16. López N, Varo N, Díez J, Fortuño MA. Loss of myocardial LIF receptor in experimental heart failure reduces cardiotrophin-1 cytoprotection. A role for neurohumoral agonists? Cardiovasc Res. 2007; 75:536–545.
17. Stanley WC. Partial fatty acid oxidation inhibitors for stable angina. Expert Opin Investig Drugs. 2002; 11:615–629.
18. Fragasso G, Palloshi A, Puccetti P, et al. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol. 2006; 48:992–998.
19. Labrou A, Giannoglou G, Zioutas D, Fragakis N, Katsaris G, Louridas G. Trimetazidine administration minimizes myocardial damage and improves left ventricular function after percutaneous coronary intervention. Am J Cardiovasc Drugs. 2007; 7:143–150.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download