Abstract
Although tuberculosis is highly prevalent worldwide, congenital tuberculosis is one of the least common manifestations of the disease. The diagnosis is usually difficult because of the non-specific clinical presentation and the lack of awareness of maternal disease prior to pregnancy and delivery. We present the case of a preterm neonate with congenital tuberculosis, born to a previously healthy mother who had developed severe disseminated tuberculosis during her pregnancy. Once the diagnosis was confirmed in the mother, the congenital infection was confirmed by isolation of Mycobacterium tuberculosis in gastric aspirates, and positive polymerase chain reaction in a cerebrospinal fluid examination. Treatment for tuberculosis with a four-drug regimen resulted in an adequate clinical response in both the mother and infant.
Tuberculosis (TB) is one of the most common and prevalent infectious diseases in the world. According to the World Health Organization, in the 90s, approximately 90 million people developed the disease and 30 million died. By the year 2010, more than 4 million new cases were reported by the 22 countries with the highest disease burden1. This increase in TB cases has been mainly in young adults with a subsequent increase of cases in women of child-bearing age123. This in turn, carries a higher risk of transmission and infection in children, who are the sentinel cases within a community because pediatric cases suggest a recent exposure to a contagious adult4.
In spite of the high prevalence of TB worldwide, few cases of congenital disease are reported: by 1984, less than 300 cases had been reported, and since then only 80 more have been reported56. In Colombia, as in other parts of the world, there are very few reported cases2789. We present a case of congenital TB acquired from a previously healthy mother who developed the disseminated disease during her pregnancy.
A 24-year-old, previously healthy mother in her second pregnancy, with negative human immunodeficiency virus testing and who worked in health-care, presented to the emergency department with fever, constitutional symptoms and preterm labor. Her initial laboratory examinations revealed leukocytosis with elevated C-reactive protein (CRP). Despite obstetrical management, she delivered vaginally a preterm infant (weight 1,730 g, length 46 cm, and Apgar score of 2 and 7, at 1 and 5 minutes, respectively). During labor and delivery, only vaginal hyperthermia and warm amniotic fluid were reported. The mother received treatment for chorioamnionitis.
The newborn infant was transferred to the neonatal unit due to mild respiratory distress and for work up of suspected sepsis. Treatment with ampicillin and gentamycin was started. Initial laboratory examinations in the newborn were normal and blood cultures were negative. He completed 7 days of antibiotics and was discharged in good condition.
In the post-partum period, the mother remained hospitalized because she developed a fever, abdominal pain, foul smelling lochia, and signs on physical examination consistent with an acute abdomen. She remained very symptomatic, in spite of antimicrobial therapy, and a computed tomographic scan of the abdomen revealed signs of peritonitis. She was taken to surgery on hospital day 8 for exploratory laparotomy with evidence of acute pelvic peritonitis with severe inflammatory and necrotic changes of the uterus, ovaries, and fallopian tubes. A hysterectomy with left salpingectomy and appendectomy were performed. During the post-operative period, she remained febrile and developed worsening respiratory distress, with bilateral alveolar infiltrates consistent with pneumonia and a small pleural effusion evidenced on the chest X-ray. Broad spectrum antibiotics were added without clinical response and on hospital day 17 she was transferred to the intensive care unit for ventilator support. At this time, histopathology from surgical samples reported chronic granulomatous inflammation with necrotic changes of the ovaries, cervix, uterus and appendix, and the acid-fast bacilli (AFB) stains (Ziehl-Neelsen) were all positive (Figure 1). On follow-up radiological images of the lungs, there were diffuse bilateral nodular infiltrates consistent with miliary TB. Treatment with a four-drug regimen for TB was started (isoniazid, rifampin, pyrazinamide, and ethambutol). Subsequently, samples from bronchoalveolar lavage were also positive for AFB and Mycobacterium tuberculosis was isolated from all the cultures. The newborn infant was readmitted to the hospital for work-up.
On admission, the baby was 19 days old, and she was in good condition, without fever, respiratory distress or hepatosplenomegaly and with adequate weight gain. The chest X-ray and laboratory tests, including tuberculin test, were all normal. Initial gastric aspirates were negative for AFB. On hospital day 7, she developed apnea and desaturations. The cell blood count showed leukocytosis (15,300/mm3), with neutrophil predominance 64%, platelets 386,000 mm3, CRP 5.3 mg/dL and on a chest X-ray, bilateral alveolar infiltrates were observed (Figure 2A). Antibiotics were started for suspected nosocomial pneumonia and cultures obtained from cerebrospinal fluid (CSF), blood, and urine (subsequently all cultures were negative and normal CSF indices). The patient developed worsening respiratory distress syndrome so gastric aspirates were obtained again that were reported AFB positive (Figure 2B). Treatment for TB with a four-drug regimen was started (isoniazid, rifampin, pyrazinamide, and amikacin). A new lumbar puncture showed normal CSF indexes, negative AFB stain, but positive polymerase chain indices (PCR) for M. tuberculosis. Subsequently, M. tuberculosis was isolated from cultures of gastric aspirates, but not from CSF cultures. A magnetic resonance imaging of the brain was normal. The mother and the neonate tests showed susceptibility to isoniazid, rifampin, ethambutol and streptomycin (first-line anti-TB drugs).
Afterward, the patient showed a steady improvement, with resolution of the fever and oxygen requirements. A follow-up chest X-ray on hospital day 30 revealed significant improvement of the lung infiltrates and follow-up gastric aspirates were negative for AFB and no mycobacteria were isolated. The patient was discharged home and completed 12 months of anti-TB therapy without complications.
Aside from mild, transient hepatotoxicity, the mother also completed her treatment and showed clinical resolution of her disease.
Congenital TB is the least frequent clinical manifestation of the disease in spite of its high prevalence around the world341011. Few cases have been reported and, therefore, many aspects of the disease course have not been described completely. Mortality from congenital TB can exceed 44%, and up to 50% of the mothers are not aware of their disease until a diagnosis is established in their newborn infants 351213. The criteria used to differentiate congenital TB from TB acquired postnatally were established by Beitzke in 193510, and subsequently reviewed by Cantwell et al.14. This last author suggests that in order to establish a diagnosis of congenital TB the child must have tuberculous lesions plus at least one of the following: (1) lesions presenting in the first week of life; (2) a primary hepatic complex or caseating hepatic granulomas; (3) TB infection of the placenta or maternal genital tract; and (4) exclusion of postnatal transmission through a study of possible contacts, including health-care personnel. Several of Cantwell's criteria were found in our patient including clinical, radiological and microbiological evidence of disseminated TB (Figure 2), demonstration of AFB by pathology and microbiology studies of the endometrium, ovaries, and cervix of the mother (Figure 1), and the exclusion of postnatal transmission because only the mother had respiratory symptoms and she remained hospitalized after birth. Some authors suggest that making the difference between congenital TB from the infection acquired postnatally would only be important from an epidemiological point of view, and propose the term perinatal TB, rather than congenital TB because the clinical presentations, treatment and prognosis are the same1012.
TB during pregnancy can be associated with concomitant bacillemia that results in infection of the maternal genital tract and placenta. Subsequently, congenital TB can result from the rupture of a placental tubercle to the fetal circulation via the umbilical vein or from aspiration or swallowing of amniotic fluid or contaminated blood secondary to maternal infection101114. Hematologic dissemination results in the formation of one or more primary complexes in the liver and lungs, while aspiration or swallowing of infected blood or amniotic fluid will result in the formation of multiple pulmonary or gastrointestinal tract primary complexes314. The bacilli remain dormant in the fetal lung and begin their multiplication after birth when oxygen saturation and circulation increase, which results in the disseminated disease15. In our patient, the most likely course was infection from ingestion of infected amniotic fluid from maternal infection of the endometrium and genital tract, since the infant did not present signs of disseminated disease (such as lymphadenopathies or hepatosplenomegaly) at birth. TB during pregnancy carries an unfavorable prognosis for the fetus, including a nine-fold risk of preterm labor, intrauterine growth retardation, low birth weight and increase in perinatal mortality347. At the same time, TB increases four times the risk of obstetric morbidity5. In our case, the mother initially presented with symptoms attributed to chorioamnionitis which subsequently progressed to a pelvic peritonitis and disseminated disease that required surgical intervention and intensive care.
The clinical manifestations of our patient coincide with those reported in the literature. Signs and symptoms developed once the newborn was readmitted for work-up after the maternal disease was suspected. Abughali et al.13, reported that the most common signs found in congenital TB are liver enlargement with or without splenomegaly, respiratory distress, fever, lymphadenopathy, irritability or lethargy, and abdominal distention1214. The average time until signs and symptoms develop was 24 days (range, 1-84 days). Central nervous system involvement is variable but can be as high as 20%. In our patient, initial CSF studies were negative, including AFB stains and cultures, but the diagnosis was finally confirmed with a positive PCR for M. tuberculosis in the CSF, which correlates with reports in the literature of patients that have central nervous system disease with normal CSF indices212. The Table 1 summarizes the main clinical findings of our patient compared to three other cases reported in Colombia.
Before isoniazid was available for the treatment of congenital TB, mortality was 100%. Since the introduction of TB drugs, mortality has decreased but still remains close to 50% because of the delay in diagnosis due to the non-specific clinical findings and the lack of awareness of maternal disease in the majority of cases21014. Recommended treatment includes a four drug anti-TB regimen for the first 2 months, followed by a two-drug regimen to complete a total of 12 months because most of the patients with congenital TB develop miliary disease and, frequently, there is central nervous system involvement.
Congenital TB continues to be an unusual diagnosis in pediatric patients. The nonspecific nature of the disease in the newborn infant and the lack of knowledge of the maternal disease prior to delivery, makes the diagnosis a clinical challenge both pre and postnatally. The disease should be suspected in neonates that present respiratory distress, fever and hepatosplenomegaly in the first 3 months of life; neonates with suspected sepsis not responding to routine antibiotic therapy in whom a bacterial or viral etiology has been excluded; neonates with radiological studies revealing a miliary pattern in the lungs and/or focal lesions of the liver and spleen; and newborn infants born to mothers with active TB5.
Figures and Tables
Figure 1
Histopathological findings in the endometrium, uterus and appendix. (A) Acid-fast bacilli (AFB) stain with Ziehl-Neelsen: AFB+ in endometrial tissue. (B) Caseating granuloma with central necrosis consistent with tuberculosis in uterus. (C) Granulomas in the appendiceal wall. (D) Necrotizing granulomas in the uterine wall.
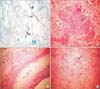
Figure 2
(A) Chest X-ray of the newborn at 4 weeks of age. There are bilateral, alveolar infiltrates suggestive of hematogenous dissemination. (B) Acid-fast bacilli+ in gastric aspirate.
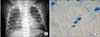
Table 1
Main clinical characteristics in four cases of congenital TB reported in Colombia

| Clinical characteristic | Cortes Buelvas et al.2 | David and Ojeda9 | Mantilla Hernandez and Cardenas8 | Present case |
|---|---|---|---|---|
| Start of symptoms, day | 28 | 30 | 41 | 26 |
| Hepatomegaly | + | NR | + | - |
| Fever | - | + | + | + |
| Respiratory distress | + | + | + | + |
| Initial lung X-ray | Normal | Abnormal | Abnormal | Normal |
| F/U X-rays | Abnormal | NR | NR | Abnormal |
| Hydrocephalus | + | - | - | - |
| Abdominal distention | + | NR | NR | - |
| Seizures | + | - | - | - |
| CSF | Normal | Normal | NR | Normal |
| Postmortem diagnosis | + | - | - | - |
| Diagnosis of maternal TB at birth | Unknown | Unknown | Unknown | Unknown |
| Preterm infants | + | + | + | + |
| Deaths | + | - | - | - |
References
1. Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med. 2012; 367:348–361.
2. Cortes Buelvas A, Osorio MA, Bolivar G, Lopez P, Palomino MF. Tuberculosis congénita. Informe de un caso con autopsia. Colomb Med. 2000; 31:185–188.
3. Ormerod P. Tuberculosis in pregnancy and the puerperium. Thorax. 2001; 56:494–499.
4. Dalamon RS, Cantelli SN, Jaroslavsky D, Bruno M, Debuh MA, Falk DJ. Tuberculosis congenita. Presentacion infrecuente de una enfermedad habitual. Arch Argent Pediatr. 2008; 106:147–150.
5. Nakbanpot S, Rattanawong P. Congenital tuberculosis because of misdiagnosed maternal pulmonary tuberculosis during pregnancy. Jpn J Infect Dis. 2013; 66:327–330.
6. Coulter JB. Perinatal tuberculosis. Ann Trop Paediatr. 2011; 31:11–13.
7. Sosa LM, Cala LL, Mantilla JC. Congenital tuberculosis associated with maternal disseminated miliary tuberculosis. Biomedica. 2007; 27:475–482.
8. Mantilla Hernandez JC, Cardenas NC. Tuberculosis congenita: reporte del primer caso en el nororiente colombiano. Medicas UIS. 2007; 20:137–142.
9. David M, Ojeda P. Tuberculosis congenita. Presentacion de caso clínico. Rev Colomb Neumol. 2004; 16:189–192.
10. Ray M, Dixit A, Vaipei K, Singhi PD. Congenital tuberculosis. Indian Pediatr. 2002; 39:1167–1168.
11. Satti KF, Ali SA, Weitkamp JH. Congenital infections. Part 2: Parvovirus, Listeria, tuberculosis, syphilis, and varicella. Neoreviews. 2010; 11:e681–e695.
12. Hageman J, Shulman S, Schreiber M, Luck S, Yogev R. Congenital tuberculosis: critical reappraisal of clinical findings and diagnostic procedures. Pediatrics. 1980; 66:980–984.
13. Abughali N, van Der Kuyp F, Annable W, Kumar ML. Congenital tuberculosis. Pediatr Infect Dis J. 1994; 13:738–741.
14. Cantwell MF, Shehab ZM, Costello AM, Sands L, Green WF, Ewing EP Jr, et al. Brief report: congenital tuberculosis. N Engl J Med. 1994; 330:1051–1054.
15. Skevaki CL, Kafetzis DA. Tuberculosis in neonates and infants: epidemiology, pathogenesis, clinical manifestations, diagnosis, and management issues. Paediatr Drugs. 2005; 7:219–234.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download