This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
Hyperthermic intraperitoneal chemotherapy (HIPEC) has been proposed as a treatment in ovarian cancer. A recently published RCT demonstrated that HIPEC prolongs disease-free survival (DFS) and overall survival (OS) in ovarian cancer. The aim of the study was to investigate oncologic results of cytoreductive surgery+HIPEC compared with cytoreductive surgery alone in advanced primary ovarian cancer with a particular attention to the pattern of recurrence.
Methods
This is a retrospective case control study with a propensity score (PS) matching of the patients. All the patients treated for primary advanced ovarian cancer who underwent interval surgery with or without HIPEC were collected; a PS was calculated in order to match cases to controls.
Results
Among 77 eligible patients 56 patients were included in the study. Preoperative patients' characteristics were homogeneous. No difference in morbidity and mortality after surgery were recorded. DFS was not different among the 2 groups (13.2 vs. 13.9 months, p=0.454) but OS was better in patients treated with HIPEC with no median reached vs. 35.5 months (p=0.048). Patients treated with cytoreductive surgery alone were more likely to have a peritoneal recurrence (43% vs. 14%).
Conclusion
HIPEC seems to affect the relapse pattern with lesser peritoneal recurrence. This difference in relapse pattern seems to affect the OS with better results in patients treated with HIPEC. Further studies are needed to confirm these findings.
Keywords: Ovarian Neoplasms, Cytoreduction, Hyperthermia, Induced, Disease-Free Survival, Neoplasm Recurrence, Propensity Score
INTRODUCTION
Epithelial ovarian cancer (EOC) is the first cause of death for gynecological cancer among women with a high percentages of patients diagnosed with disseminated disease at diagnosis (International Federation of Gynecology and Obstetrics [FIGO] stage IIIC–IV) [
1]. In ovarian cancer, the peritoneum is most often involved with spreading of tumoral cells from the ovary into the peritoneal cavity with dissemination and implantation. Due to its nature, the peritoneum is poorly reached by systemic chemotherapy due to the plasma-peritoneal barrier [
2345]. For these reasons intraperitoneal chemotherapy was introduced with promising results: the administration of drug directly into the peritoneal cavity allows an effective dose delivered to the tumor site without reaching plasma toxicity levels [
4678]. Moreover, chemotherapeutics could be delivered associated with hyperthermia (hyperthermic intraperitoneal chemotherapy [HIPEC]) increasing the effect of the drug with the heat [
9].
Therefore, the interest in HIPEC in ovarian cancer in the last years has raised considerably with lots of studies published investigating this issue with promising results [
10111213]. Recently were published the results of the first randomized controlled trial investigating the effect of HIPEC in primary EOC: 245 patients were randomized to receive HIPEC or cytoreductive surgery; the disease-free survival (DFS) and overall survival (OS) were significantly higher in patients who received HIPEC (10.7 vs. 14.2 months, p=0.003 and 33.9 vs. 45.7 months p=0.02, respectively). [
14]. These results confirm the results of the previously published trial on HIPEC in recurrent ovarian cancer that demonstrated a better OS (26 vs. 13 months, p=0.006) compared to standard treatments [
15]. At the moment, another randomized trial is now undergoing investigating the role of HIPEC after neoadjuvant chemotherapy (NACT) in primary ovarian cancer (the CHORINE Trial,
NCT01628380) [
16].
The aim of the present study was to investigate the role of cytoreductive surgery+HIPEC in primary EOC after NACT compared to the cytoreductive surgery alone with a focus on the effect of HIPEC on the site of recurrence. In order to increase the quality of the results and the derived level of evidence and to reduce the selection bias, the study was designed as a case control study with a propensity score (PS) based matching of the patients.
MATERIALS AND METHODS
This is a retrospective case control study derived from a prospectively collected database of patients treated for advanced EOC at the Papa Giovanni XXIII Hospital, Bergamo, Italy, from January 2010 to June 2016. The study protocol was reviewed and approved by the local Ethical Commission.
At our institution, NACT with interval surgery is the standard treatment of patients with primary EOC; all the patients with a diagnosis of primary EOC who underwent NACT with combination of carboplatin (CBDCA) and paclitaxel were included in the study. Patients were assigned to treatment after a multidisciplinary discussion among general surgeon, gynecologist, and medical oncologist. Patients were divided in 2 groups based on type of intervention received after NACT: only cytoreductive surgery or cytoreductive surgery+HIPEC. After NACT patients were reevaluated and the chemosensitivity was assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST) criteria and the clinical response: all the patients were treated even in case of progression of the disease. HIPEC was performed only after optimal cytoreductive surgery as an open procedure with the coliseum technique [
17] using cisplatin (100 mg/m
2) and paclitaxel (175 mg/m
2) at the temperature of 41.5°C for 90 minutes; in case of bowel resection the anastomosis was performed after the chemohypertermic phase.
According to the protocol, after surgery and post-operative period, patients were referred to the medical oncologic staff to continue systemic chemotherapy and were followed after the end of chemotherapy every 4 months for the first 2 years and every 6 months for further 3 years.
For each patient was evaluated and recorded preoperative data, intraoperative and post-operative course data and follow-up.
Preoperative data included: age at diagnosis, number of cycle of NACT, sensitivity to chemotherapy defined on the basis of the clinical response to NACT according to the RECIST criteria, number of days between last NACT cycle and surgery, stage of disease according to FIGO, histologic type and grade.
Intraoperative data include: peritoneal cancer index (PCI), completeness of cytoreduction (CC) assessed by measuring the size of the residual peritoneal implants following surgery and assigning the score as follows: CC0, no residual disease; CC1, residual nodules measuring less than 2.5 mm; CC2, residual nodules measuring between 2.5 mm and 2.5 cm; or CC3, residual nodules greater than 2.5 cm [
18]; length of stay, complications and perioperative death. Follow-up data include duration of follow-up, adjuvant chemotherapy (ACT), any relapse with location of the relapse and relapse pattern divided into systemic, peritoneal or both; treatment of the relapse and further chemotherapy lines and date of death.
1. Statistical analysis
In order to reduce the variability and heterogeneity among included patients and reduce the selection bias a PS model [
1920] was calculated considering as covariates the age of patients, FIGO stage, sensitivity to chemotherapy, PCI, and CC status. Patients treated with cytoreductive surgery+HIPEC were matched in a proportion of 1:1 with patients treated with cytoreductive surgery alone using the nearest neighbor based on the PS with a caliper of 0.2.
Continuous variables were reported as mean (standard deviation) or median (interquartile range) and compared with the Mann-Whitney U test after the study for skewness as appropriate; categorical variables were expressed as proportion and percentages and were compared with the Pearson's χ2 test. Survival analysis were performed with the Kaplan-Meier method and results were showed as median with 95% confidence interval and were compared with the log-rank test. Statistical analyses were performed with SPSS 24 (IBM SPSS Statistics for Windows, version 24.0; IBM Corp., Armonk, NY, USA).
RESULTS
During the study period 77 patients with primary advanced EOC underwent NACT and were included in the study. After the PS based matching 56 patients were included for the final analysis, 28 treated with cytoreductive surgery+HIPEC and 28 treated with cytoreductive surgery alone.
Table 1 shows in detail population's characteristics before and after PS based matching. Patients treated with chemotherapy response score+HIPEC were operated after a significantly smaller time interval (27 vs. 56 days, p=0.015) after NACT compared with controls, also with a lesser number of chemotherapy cycles (4.1 vs. 4.6, p=0.014). Intraoperative characteristics did not differ significantly with a similar burden of disease (PCI and stage), similar histologic types and similar results in term of CC (p=1.000); no differences were found in post-operative course with a similar length of stay and comparable severe complications' rate. No peri-operative death was recorded. Severe acute renal failures were recorded in one patient in the HIPEC group.
Table 2 shows study's results in detail.
Table 1
Patients' characteristics before and after PS matching

|
Characteristics |
All |
After PS matching |
|
Cytoreductive surgery |
Cytoreductive surgery+HIPEC |
p |
Cytoreductive surgery |
Cytoreductive surgery+HIPEC |
p |
|
No. of patients |
49 |
28 |
|
28 |
28 |
|
|
Age |
63.48 (11.85) |
58.99 (8.42) |
0.082 |
61.55 (10.38) |
58.99 (8.42) |
0.317 |
|
NACT No. of cycles |
5.02 (1.64) |
4.14 (1.20) |
0.016 |
4.69 (1.25) |
4.14 (1.20) |
0.017 |
|
Sensitivity to NACT |
25 (89.3) |
43 (87.8) |
0.841 |
25 (89.3) |
25 (89.3) |
1.000 |
|
Days from NACT to surgery |
52.27 (23.30) |
27 (1.64) |
0.025 |
56.96 (27.78) |
27 (1.64) |
0.007 |
|
Stage |
|
|
0.221 |
|
|
0.537 |
|
IIIC |
32 (35.3) |
22 (78.6) |
20 (71.4) |
22 (78.6) |
|
IV |
17 (34.7) |
6 (21.4) |
8 (28.6) |
6 (21.4) |
|
Grade |
|
|
0.536 |
|
|
1.000 |
|
G1 |
1 (2) |
1 (3.6) |
1 (3.6) |
1 (3.6) |
|
G2 |
3 (6.1) |
1 (3.6) |
1 (3.6) |
1 (3.6) |
|
G3 |
44 (89.8) |
26 (92.9) |
26 (92.9) |
26 (92.9) |
|
Histologic type |
|
|
0.091 |
|
|
0.130 |
|
Serous |
47 (95.9) |
25 (89.3) |
27 (96.4) |
25 (89.3) |
|
Endometriod |
0 |
3 (10.7) |
0 |
3 (10.7) |
|
Clear cells |
1 (2) |
0 |
1 (3.6) |
0 |
|
PCI |
6.43 (6.98) |
8.25 (6.79) |
0.270 |
6.36 (6.19) |
8.25 (6.79) |
0.281 |
|
CC |
|
|
0.409 |
|
|
1.000 |
|
CC0 |
39 (79.6) |
26 (92.9) |
23 (92.9) |
23 (92.9) |
|
CC1 |
4 (8.2) |
1 (3.6) |
1 (3.6) |
1 (3.6) |
|
CC2 |
3 (6.1) |
1 (3.6) |
0 |
0 |
|
CC3 |
3 (6.1) |
0 |
0 |
0 |
Table 2
Study results

|
Characteristics |
Cytoreductive surgery |
Cytoreductive surgery+HIPEC |
p |
|
No. of patients |
28 |
28 |
|
|
Lenght of surgery |
194 (85.15) |
533 (82.22) |
<0.001 |
|
Major complications |
5 (17.9) |
7 (25) |
0.515 |
|
Peri-operative death |
0 |
0 |
|
|
ICU lenght of stay |
2.18 (6.45) |
2.88 (9.08) |
0.863 |
|
Lenght of stay |
14 (10–25) |
15 (10–25) |
0.367 |
|
ACT |
27 (96.4) |
21 (75) |
0.022 |
|
Days from surgery to ACT |
37 (30–50) |
49 (42–54) |
0.004 |
|
Number of completed cycles of ACT |
3.67 (1.38) |
3.14 (1.01) |
0.153 |
|
Follow-up |
28 (12–45) |
34 (16–54) |
0.579 |
|
Recurrence |
23 (82.1) |
21 (75) |
0.515 |
|
Recurrence location |
|
|
0.097 |
|
Peritoneum |
10 (43) |
3 (14) |
|
Systemic |
7 (30) |
13 (62) |
|
Systemic+peritoneal |
6 (26) |
5 (24) |
|
DFS |
13.23 (10.64–15.81) |
13.96 (7.91–20.01) |
0.454 |
|
Further CT after relapse |
|
|
0.351 |
|
0 |
7 (30.4) |
3 (14.3) |
|
1 |
4 (17.4) |
8 (38.1) |
|
2 |
6 (26.1) |
5 (23.8) |
|
3 |
5 (21.7) |
2 (9.5) |
|
4 |
1 (4.3) |
2 (9.5) |
|
5 |
0 |
1 (4.8) |
|
Further surgery after relapse |
4 (17.3) |
0 |
0.134 |
|
OS |
32.53 (21.87–43.19) |
- |
0.048 |
After surgery, 75% of patients treated with cytoreductive surgery+HIPEC underwent ACT after and median of 49 days from surgery, receiving a median of 3 cycles compared with the 96% of patients treated with only cytoreductive surgery (p=0.022) who underwent a median of 3 cycles after a median of 37 days (p=0.004).
Median follow-up was 28 (12–45) months and 34 (16–54) months in cytoreductive surgery alone group and cytoreductive surgery+HIPEC group respectively (p=0.579).
In cytoreductive surgery+HIPEC group, 75% of patients had a relapse after a median time of 13.96 (7.9–20.01) months compared with 82% in the control group after a median of 13.23 (10.64–15.81) months (p=0.515) (
Fig. 1). The treatment of the recurrence did not differ among the 2 group with a similar number of chemotherapy schemes; target therapies with bevacizumab or poly (ADP-ribose) polymerase inhibitor (PARPi) were not administered in the study population during the study period.
Fig. 1
Kaplan-Meier curves of DFS in patients treated with cytoreductive surgery alone and with cytoreductive surgery+HIPEC.
DFS, disease-free survival; HIPEC, hyperthermic intraperitoneal chemotherapy.

Recurrence of the disease was located only in the peritoneum in 14% of patients treated with cytoreductive surgery+HIPEC compared to 43% in the control group; in 62% of patients treated with HIPEC was recorded a systemic relapse with exclusion of the peritoneum compared to 30% in the control group (
Fig. 2). Analyzing the effect of relapse pattern on OS the involvement of the peritoneum as a location of recurrence was associated with a worse prognosis (
Fig. 3) (p=0.026).
Fig. 2
Site of relapse among patients treated with cytoreductive surgery alone and with cytoreductive surgery+HIPEC.
HIPEC, hyperthermic intraperitoneal chemotherapy.

Fig. 3
Kaplan-Meier curves of OS based on site of relapse.
OS, overall survival.
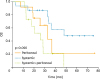
OS differs significantly among the 2 treatments (p=0.048) with a median of 32.53 months in control group and no median reached in the HIPEC group after a median follow-up time of 43 months (
Fig. 4).
Fig. 4
Kaplan-Meier curves of OS in patients treated with cytoreductive surgery alone and with cytoreductive surgery+HIPEC.
HIPEC, hyperthermic intraperitoneal chemotherapy; OS, overall survival.

DISCUSSION
HIPEC in ovarian cancer has been demonstrated to achieve better results than standard treatments in ovarian cancer, as shown in the recently published trial by van Driel and colleagues [
14]. The present study confirms those results showing that cytoreductive surgery combined with HIPEC after NACT in advanced EOC prolongs OS. The 2 groups of patients after the PS based matching were homogeneous and comparable. Patients received similar preoperative treatment and had a comparable burden of disease (similar PCI) and debulking surgery achieved same results in the 2 groups. The introduction of HIPEC after cytoreductive surgery significantly prolonged length of surgery but did not affect significantly the rate of severe complications and the length of hospital stay. No perioperative mortality was observed during the study period. Follow-up results did not show any difference in recurrence rate with similar DFS intervals (13.23 vs. 13.96 months, p=0.454). Surprisingly, in patients treated with cytoreductive surgery+HIPEC the OS was significantly better compared to the group of cytoreductive surgery alone, despite the similar preoperative characteristics and the lower proportion of patients that received ACT in the HIPEC group and, above all, despite the similar recurrence rate.
Analyzing the treatment received after the relapse, the 2 groups of patients were still similar with a similar proportion of patients that received further chemotherapy lines and no significant difference in patients treated with further surgery. This difference in OS with a similar DFS moved us to study the reason for this unexpected finding.
A possible interpretation of these data could be individualized in the relapse pattern into the 2 groups. In our study, the site of the recurrence affected significantly the OS with a better prognosis if the relapsed disease did not affect the peritoneum (p=0.026).
Because of relatively small number of included patients, the 2 groups had an evident different distribution of the site of recurrence with the involvement of the peritoneum more than double in the cytoreductive surgery group compared with cytoreductive surgery+HIPEC group. Actually, at the best of our knowledge there are no similar results published in literature. Therefore, these findings suggest us some considerations.
Peritoneal dissemination of disease, not only in ovarian cancer but above all in gastrointestinal cancers has been historically considered as a metastatic and incurable disease associated with a poor prognosis. Ovarian cancer is a particular exception where the presence of peritoneal carcinomatosis is not considered as a stage IV disease with metastasis but remain a locally advanced disease, due to the high chemosensitivity of this neoplasm.
However, the peritoneum, with its particular anatomy and physiology [
21] did not allow to systemic chemotherapeutic drugs to reach a proper concentration and to penetrate effectively all the disease. For these reasons, intraperitoneal chemotherapy was introduced in clinical practice, in order to treat directly the neoplastic implantation into the peritoneum layer bypassing the plasma-peritoneum barrier that prevent an incisive effect of systemic drugs.
At the light of these considerations, the different relapse pattern of the 2 groups could be interpreted as the reason of better survival in patients treated with HIPEC despite the homogeneous characteristics before surgery and the similar treatments received after recurrence. The lower rate of peritoneal involvement and, on the other hand, the higher rate of systemic recurrence of disease (lymphatic and solid organ metastasis) could justify a more incisive effect of the treatment of recurrent disease: the same pharmacological scheme administered for a lymphatic localization could not have the same efficacy on a peritoneal localization.
Although these are only considerations, the introduction of HIPEC after cytoreductive surgery seems to act into the peritoneal cavity removing all the disease, macro and microscopic, and reducing considerably the rate of recurrence in the abdomen more than the only cytoreductive surgery. In fact, even the CC was similar in the 2 groups the surgeon can remove only the visible and macroscopic disease with the possibility to leave some microscopic implantation or free cell into the peritoneal cavity. In the group of patients treated with only cytoreductive surgery the rate of peritoneal relapse was more than double than in patients treated with HIPEC (43% vs. 14%). Consequently, even if the recurrence rate in the 2 groups was similar, the different site of recurrence seems to have affected significantly the effect of treatment with a better OS in patients without relapse into peritoneum, i.e., patients treated with cytoreductive surgery+HIPEC.
Since the 2 groups of patients received a similar number and schemes of treatment after the recurrence our results could demonstrate that recurrence into the peritoneum has a lesser sensitivity and a worse response to standard treatment, the systemic chemotherapy.
This study has the great limit to be a retrospective not randomized study including a relatively small number of patients; on the other hand, the study was designed as a case control study with a PS based matching of the patients in order to reduce to the minimum the difference among patients and to reduce the selection bias, to simulate a randomized setting [
20].
The available and the undergoing randomized trials investigating the role of intraperitoneal chemotherapy in ovarian cancer unfortunately are designed and are focused on the endpoint of the DFS and the OS and no data are now available on the recurrence pattern. These new findings on recurrence pattern after HIPEC should be confirmed in larger and well-designed studies.
In conclusion, the present study shows that relapse pattern in advanced EOC seems to be influenced by cytoreductive surgery+HIPEC, with lower rate of peritoneal involvement. The relapse pattern seems to affect also the OS with better results in patients without peritoneal involvement. Further well-designed studies are needed to confirm these findings.














