INTRODUCTION
MATERIALS AND METHODS
RESULTS
Table 1
Baseline characteristics of all 35 patients
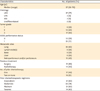
Table 2
Maximum grade toxicity recorded per patient (n=35)

Journal List > J Gynecol Oncol > v.29(1) > 1093900


Funding This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A3B04031900).
Author Contributions
Conceptualization: K.H.J., P.S.H.
Data curation: K.Y., L.S.J., L.J.
Formal analysis: K.H.J., L.S.J., P.S.H.
Funding acquisition: K.H.J.
Investigation: K.H.J., L.S.J., P.S.H.
Methodology: K.Y., L.J.
Project administration: P.S.H.
Resources: K.Y., L.S.J., L.J., P.S.H.
Software: K.Y., L.S.J., L.J.
Supervision: P.S.H.
Validation: K.H.J., L.J., P.S.H.
Visualization: L.S.J., L.J.
Writing - original draft: K.H.J., K.Y.
Writing - review & editing: K.H.J., P.S.H.
Hyun-Jun Kim 
https://orcid.org/0000-0002-3260-6280
Youjin Kim 
https://orcid.org/0000-0002-9108-5286
Su Jin Lee 
https://orcid.org/0000-0003-3405-9935
Jeeyun Lee 
https://orcid.org/0000-0001-9536-8059
Se Hoon Park 
https://orcid.org/0000-0001-5084-9326