This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
In 2014 World Health Organization criteria, seromucinous carcinoma was defined as a new histological subtype in ovarian carcinomas, but “seromucinous carcinoma” was not defined in endometrial carcinomas. The aim of this study was to identify seromucinous carcinoma resembling ovarian seromucinous carcinoma in endometrial carcinomas, and to evaluate the clinical significance for prognoses of the patients.
Methods
Central pathological review was conducted for patients with endometrioid carcinoma of the endometrium treated by primary surgery at our hospital between 1990 and 2013.
Results
Among 340 cases included in the study, no case had all tumor cells resembling ovarian seromucinous carcinoma in all specimens, and 31 cases (9.1%) had seromucinous component in combination with endometrioid carcinomas. Immunohistochemical analysis revealed seromucinous component had positive reactivity for cytokeratin (CK) 7, and negative reactivity for CK20 and caudal type homeobox 2 (CDX2) in all cases. Seromucinous component showed lower immunoreactivity of estrogen receptor and progesterone receptor, compared with endometrioid carcinoma component. Progression-free survival of the cases with seromucinous component was better than those without seromucinous component (p=0.049).
Conclusion
Seromucinous component was identified in approximately 10% of endometrioid carcinoma, and could be a histological predictor for prognosis.
Keywords: Endometrial Neoplasms, Seromucinous, Endometrioid Carcinoma, Disease-Free Survival
INTRODUCTION
Recently, the incidence of endometrial carcinomas has been increasing [
1]. In endometrial carcinomas, histological subtype was one of important factors to predict the prognosis in addition to International Federation of Obstetrics and Gynecology (FIGO) stage and grade [
2]. According to the 2014 World Health Organization (WHO) criteria, histological subtypes of endometrial carcinomas were categorized into 9 subtypes; endometrioid carcinoma, mucinous carcinoma, serous endometrial intraepithelial carcinoma, serous carcinoma, clear cell carcinoma, neuroendocrine carcinoma, mixed cell adenocarcinoma, undifferentiated carcinoma, and dedifferentiated carcinoma [
3].
In ovarian cancers, seromucinous carcinoma has been recently recognized as one of new histological subtypes and were listed in 2014 WHO criteria [
34]. The pathological findings of ovarian seromucinous carcinomas were defined as tumors containing endocervical mucinous and serous type cells. Seromucinous tumors are generally papillary and display epithelial stratification closely resembling serous tumors. Its mitosis index was variable but tended to be low, and the common pattern of invasion was cribriform and expansile. In some cases, tumors had foci of endometrioid differentiation including squamous differentiation [
3]. Most of ovarian seromucinous carcinomas were stage I diseases, and the prognosis of the tumor was excellent [
5]. Coexistence of seromucinous component in endometrial carcinomas has not even been evaluated yet.
Herein, the objective of this study is to examine whether there were histological subtype resembling ovarian seromucinous carcinoma or not, and to evaluate the association between seromucinous component and clinico-pathological findings in endometrial carcinomas.
MATERIALS AND METHODS
1. Pathological review and definition
All hematoxylin and eosin slides obtained from materials obtained at surgery were used. Central pathological review and were re-diagnosis was conducted for all cases by 2 observers (M.M. and H.T.) according to 2014 WHO criteria. In our study, seromucinous carcinoma in endometrial carcinoma was defined as ovarian seromucinous carcinoma in 2014 WHO criteria. Briefly, seromucinous carcinoma in endometrial carcinoma was serous like and endocervical-type mucinous cells. Serous like cells have low mitotic index, and is clearly different entity from endometrial serous carcinomas. Seromucinous component was frequently complicated with squamous differentiation. Even a small foci resembling ovarian seromucinous carcinoma in endometrial endometrioid carcinoma was defined as seromucinous component.
2. Patients
Patients with endometrioid carcinoma treated by primary surgery including hysterectomy and bilateral salpingo-oophorectomy at our hospital between 1990 and 2013 were identified. Exclusion criteria were as follows: the cases with the other histology expect endometrioid carcinomas, the cases complicated with other carcinomas such as ovarian carcinoma. Our study was approved by the Institutional Review Board of National Defense Medical College.
3. Immunohistochemistry (IHC) staining
For IHC staining, we used progesterone receptor (PR) (SP1, ready to use; Ventana, Tucson, AZ, USA), mouse monoclonal antibody for estrogen receptor (ER) (PgR636, ready to use; Dako, Carpinteria, CA, USA), mouse monoclonal antibody for cytokeratin (CK) 7 (12/30, dilution 1:200; Dako), mouse monoclonal antibody for CK20 (Ks20.8, dilution 1:100; Dako), and mouse monoclonal antibody for caudal type homeobox 2 (CDX2 [AMT28], dilution 1:50; Novocastra, Newcastle upon Tyne, UK). Slides were deparaffinized in xylene and hydrated with alcohol. Endogenous peroxidase activity was blocked by 0.3% hydrogen peroxide/methanol. Using ER, PR, and CDX2 antibody, antigen retrieval was omitted. Using CK7 and CK20 antibody, antigen retrieval with proteinase K (ready to use; DAKO) was performed for 15 minutes. The slides were incubated at 4°C overnight with primary antibodies and reacted with the DAKO EnVision+ system-HRP labeled polymer as secondary antibody for 30 minutes at room temperature. Specific antigen-antibody reactions were visualized with 0.2% diaminobenzidine tetrahydrochloride and hydrogen peroxide, and counterstained with Mayer's hematoxylin. For each antibody, negative control studies were performed without the primary antibody.
4. Interpretation of IHC staining
Two observers (M.M. and H.T.) independently evaluated and interpreted the results of IHC staining without knowledge of the clinical data of each patient. During the course of interpretation of IHC, a multiviewer microscope was not provided and any discrepancies between the 2 observers were resolved by discussion.
For interpretation of the IHC activities of ER and PR, the percentage of positive cells was scored as follows: 1) less than 1% of the nuclei stained; 2) 1% to 10% of nuclei stained; 3) 10% to 33% of the nuclei stained; 4) 34% to 66% of nuclei stained; and 5) more than 67% of the nuclei stained. The staining intensity was scored as follows: 0, absent; 1, weak; 2, strong; 3, very strong. The sum of both parameters was defined as the IHC score. The evaluation of ER and PR expression for 2 components of endometrioid carcinoma and seromucinous carcinoma was separately done.
For the evaluation of IHC activities of CK7, CK20, and CDX2, more than 10% positive cell was defined as positive and other cases was defined as negative regardless of any intensity.
5. Statistical analysis
The χ2 test, Fisher's exact test, and Mann-Whitney U test for unpaired data were used for statistical analysis. A p-value of <0.05 was considered statistically significant. A p-value ranging from 0.05 to 0.10 was considered statistically marginal significant. The Stat View ver. 5.0 (SAS Institution Inc., Cary, NC, USA) was used for statistical analysis.
RESULTS
During study periods, 373 cases were identified. Through the central pathological review, 33 cases were excluded and 340 cases were included in our study. The reasons of exclusion were as follows: 9 cases with mixed carcinoma, 7 cases with serous carcinoma, 3 cases with carcinosarcoma, 1 case with clear cell carcinoma, and 12 cases with ovarian cancer, and 1 case with cervical carcinoma (
Fig. 1). The median period of follow-up was 63.5 months.
Fig. 1

Among a total of 340 cases enrolled in this study, 31 cases (9.1%) had seromucinous component (
Fig. 2). There were no cases with all tumor cells resembling ovarian seromucinous carcinoma in whole specimen. Among 31 cases with seromucinous component, 27 cases had grade 1 endometrioid carcinoma, and 4 cases had grade 2 endometrioid carcinoma. No cases with grade 3 endometrioid carcinoma had seromucinous component.
Fig. 2
Representative images of seromucinous component resembling ovarian seromucinous carcinoma in endometrioid endometrial carcinoma. (A) Endocervical-type mucinous tumor and squamous differentiation (×20). (B) Cancer cells containing endocervical mucinous type (×40). (C) Squamous differentiation in seromucinous component. Neutrophils invading tumors are present (×40).

IHC analyses were shown in
Fig. 3. All cases with seromucinous component had positive reactivity for CK7, and negative reactivity for CK20 and CDX2. Similarly, all cases with endometrioid carcinoma component had positive reactivity for CK7, and negative reactivity for CK20 and CDX2. The average scores of ER expression in seromucinous component and endometrioid carcinoma component were 2.58 and 5.61, respectively. Also, the average scores of PR expression in seromucinous component and endometrioid carcinoma component were 2.29 and 5.06, respectively. Immunoreactivities of both ER and PR in seromucinous component were significantly lower than those in endometrioid carcinoma component (p<0.010, p<0.010,
Fig. 4).
Fig. 3
Representative images of IHC analyses. (A) IHC stains of seromucinous component (×40). (B) IHC stains of endometrioid component (×40). IHC evaluation was undergone using antibodies against ER, PR, CK7, CK20, and CDX2. Both components in all cases had positive immunoreactivity of CK7, and negative immunoreactivity for CK20 and CDX2. Expressions of ER and PR in seromucinous component were lower than those of endometrioid component.
CK, cytokeratin; CDX, caudal type homeobox; ER, estrogen receptor; IHC, immunohistochemistry; PR, progesterone receptor.
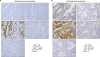
Fig. 4
Average scores of IHC reactivity. Average scores IHC reactivity of ER (A) and PR (B) in seromucinous component were lower compared with those in endometrioid component (p<0.01 and p<0.01, respectly).
ER, estrogen receptor; IHC, immunohistochemistry; PR, progesterone receptor.

The characteristics of the patients according to presence or absence seromucinous component were listed in
Table 1. There were no statistical significances of the clinico-pathological factors. The only one case with seromucinous component in endometrioid carcinoma grade 1 developed recurrence at vaginal stump after 62 months from primary surgery. Recurrent tumors were surgically resected in this case, and recurrent tumor had endometrioid carcinoma only. The case is now alive without recurrence of the disease. Progression-free survival of the cases with seromucinous component was better than that without seromucinous component (p=0.049,
Fig. 5).
Table 1
Characteristics of the patients with endometrioid carcinoma of the endometrium with or without seromucinous component

|
Variables |
Endometrioid carcinoma with seromucinous component (n=31) |
Endometrioid carcinoma without seromucinous component (n=309) |
p-value |
|
Age (yr) |
58 (41–77) |
58 (29–85) |
0.620 |
|
BMI (kg/cm2) |
23.9 |
23 |
0.470 |
|
ECOG performance status |
|
|
0.990 |
|
0 |
30 (97) |
294 (95) |
|
≥1 |
1 (3) |
15 (5) |
|
Grade |
|
|
<0.010 |
|
1 |
27 (87) |
195 (63) |
|
2 |
4 (13) |
63 (20) |
|
3 |
0 |
51 (17) |
|
Myometrial invasion |
|
|
0.100 |
|
≥1/2 |
5 (16) |
98 (32) |
|
<1/2 |
26 (84) |
211 (68) |
|
Cervical involvement |
|
|
0.620 |
|
Positive |
3 (9) |
31 (10) |
|
Negative |
28 (91) |
278 (90) |
|
Lymph-vascular invasion |
|
|
0.700 |
|
Positive |
10 (32) |
114 (37) |
|
Negative |
21 (68) |
195 (63) |
|
Peritoneal washing cytology |
|
|
0.160 |
|
Positive |
5 (16) |
23 (7) |
|
Negative |
26 (84) |
286 (93) |
|
Lymph node metastasis |
|
|
0.170 |
|
Positive |
1 (3) |
31 (10) |
|
Negative |
28 (91) |
227 (73) |
|
Not evaluable |
2 (6) |
51 (17) |
|
Ovarian metastasis |
|
|
0.990 |
|
Positive |
0 |
6 (2) |
|
Negative |
31 (100) |
303 (98) |
|
Metastasis beyond pelvis |
|
|
0.990 |
|
Positive |
0 |
8 (3) |
|
Negative |
31 (100) |
301 (97) |
|
The mode of uterine resection |
|
|
0.570 |
|
Extended hysterectomy |
15 (48) |
171 (55) |
|
Simple hysterectomy |
16 (52) |
138 (45) |
|
Lymphadenectomy |
|
|
0.190 |
|
Done |
29 (94) |
258 (83) |
|
Not done |
2 (6) |
51 (17) |
|
Adjuvant therapy |
|
|
0.930 |
|
Chemotherapy |
12 (39) |
126 (42) |
|
Radiation |
0 |
7 (2) |
|
Chemotherapy and radiation |
0 |
7 (2) |
|
Not done |
19 (61) |
169 (54) |
|
Recurrence |
|
|
0.060 |
|
Yes |
1 (3) |
49 (16) |
|
No |
30 (97) |
260 (84) |
Fig. 5
Progression-free survival of the patients with endometrioid carcinoma with or without seromucinous component. Progression-free survival of the patients with seromucinous component was better than those without seromucinous component (p=0.049).

DISCUSSION
In our study, a total of 31 cases (9.1%) with seromucinous component was identified in endometrioid carcinomas: 27 cases (87%) with grade 1 endometrioid carcinoma, and 4 cases (13%) with grade 2 endometrial carcinoma. No cases with grade 3 endometrioid carcinoma had seromucinous component. Seromucinous component was identified in 12% (27/222 cases) in grade 1 endometrioid carcinoma, 6% (4/67 cases) in grade 2 endometrioid carcinoma, and 0% (0/51 cases) grade 3 endometrioid carcinoma. Endometrial carcinoma, particularly grade 1 endometrioid carcinoma, was well-known to differentiate into several types: mucinous, squamous, or tubal differentiation [
6]. Thus, it is possible that seromucinous component was frequently discovered in grade 1 endometrioid carcinoma.
In our study, IHC analysis demonstrated seromucinous component had positive immunoreactivity for CK7 and negative immunoreactivity for CK20 and CDX2 in all cases. In general, ovarian seromucinous carcinoma showed positive reaction for CK7, and negative reaction for CK20 and CDX2 [
7], which was similar to immunoreactive pattern of endometrial seromucinous component in our study. These results suggested this seromucinous component had phenotype of endocervical-type, and did not show phenotype of intestinal type. On the other hand, our study demonstrated seromucinous component had lower immunoreactivity for ER and PR than endometrioid carcinoma component, which indicated seromucinous component was a distinguishing entity from endometrioid component. However, previous reports showed ovarian seromucinous carcinoma was characterized by frequent expression of ER and PR [
57]. Thus, the profile of hormone receptor expressions might be different between seromucinous component in endometrial carcinoma and ovarian seromucinous carcinoma. There were various differentiations such as the squamous, mucinous, secretory, and ciliated cell components in endometrioid carcinomas. Comparison of the endometrioid glandular component with the squamous, mucinous, secretory, and ciliated cell components showed that ER and PR expressions were generally higher in the glandular component compared with the various differentiated components [
8]. In our study, most of the cases had endocervical-type mucinous and squamous differentiation as seromucinous component. Seromucinous component in endometrial carcinomas could be a mixture of several morphological subtypes including various differentiations.
In our study, there were no differences of clinico-pathological factors of the cases with or without seromucinous component, and coexistence of seromucinous component in endometrioid carcinoma was a better prognostic factor. Seromucinous component was more frequently discovered in grade 1 endometrioid carcinoma, suggesting seromucinous component was one morphological subtype of well differentiated adenocarcinoma of uterine corpus. Also, the prognosis of ovarian seromucinous carcinoma was quite good [
5]. Therefore, the impact for the prognosis of seromucinous component in endometrioid carcinoma might be similar to ovarian seromucinous carcinoma.
Various cellular changes such as mucinous, tubal, eosinophilic/oncocytic, and squamous differentiation might potentially overlap with seromucinous component detected in the present study. A recent paper suggested that the morphologic diagnosis of seromucinous carcinoma was not so reliable and that approximately 70% of the cases were reclassified into endometrioid carcinomas according to integrating evaluation using morphology, IHC, and genotyping [
9]. So, further studies for integrating evaluation methods might be needed to confirm the diagnoses of seromucinous component in endometrial cancers. However, in our study, endometrioid carcinomas complicated with seromucinous component was identified as a better prognostic factor. Thus, to recognize seromucinous component could facilitate a tailor-made treatment modality for endometrioid carcinomas.
The limitations of our study included a small sample size treated at a single-institution, and a retrospective analysis. Further studies with a large sample size and multi-institutional analysis is needed to confirm clinical significance of seromucinous component in endometrial carcinomas.
In conclusion, seromucinous component was identified in approximately 10% of endometrioid carcinomas of the endometrium. Expressions of hormone receptors were lower in seromucinous component compared with endometrioid carcinoma component. The patients with seromucinous component in endometrioid carcinomas had better progression-free survival. Although further studies are needed to confirm the present results, coexistence of seromucinous component could be a histological profile predictor for better prognoses in endometrial carcinomas.








 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download