To the Editor,
In Japan, the incidence of cervical cancer among young women has been steadily increasing, and 44% of women with cervical cancer are younger than 50 years of age and present with stage I–II disease [
123]. Young Japanese women in this disease group often undergo radical hysterectomy and pelvic lymphadenectomy [
2], however, the current evidence-based guidelines per the Japan Society of Gynecologic Oncology (JSGO) lack a solid recommendation regarding oophorectomy vs. ovarian conservation at the time of surgical treatment for early-stage cervical cancer [
3].
Ovarian conservation in young women benefits many aspects of health including providing long-term protection against cardiovascular disease morbidity and mortality as well as improving bone health [
4]. In a recent population-based analysis, the ovarian conservation rate for young women with stage IB cervical cancer in the United States was reported to be around 40%, and that cervical cancer-specific survival of young women who had ovarian conservation at hysterectomy was not inferior compared to those who received oophorectomy [
5]. To date, there is little data available regarding the incidence and outcome of young women in which the ovaries were conserved at the time of radical hysterectomy for early-stage cervical cancer in Japan. The objective of the study was to examine the incidence of young women who had ovarian conservation at radical hysterectomy for clinical stage IB–IIB cervical cancer. Additionally, survival outcomes of women who had ovarian conservation were compared to those who had oophorectomy.
We analyzed a previously organized database for a large-scale nation-wide retrospective study examining women with surgically-treated clinical stage IB–IIB cervical cancer between January 2004 and December 2008 from 116 Japan Gynecologic Oncology Group (JGOG) participating institutions (data acquisition between October 2012 and February 2013). Institutional review board approval was obtained for this study. Women younger than 50 years of age with known oophorectomy status at the time of radical hysterectomy were eligible for the analysis. Information abstracted from the database for this study included patient age, clinical stage, surgical-pathological factors (histology subtypes, tumor size, parametrial tumor involvement, deep stromal invasion defined as outer half, uterine corpus tumor invasion, lymphovascular space invasion [LVSI], and presence of malignant cells in peritoneal washing), and survival outcomes (disease-free survival [DFS] and cause-specific survival [CSS] from cervical cancer).
The association of ovarian conservation and patient/tumor factors were assessed. Secondarily, the association of ovarian conservation and survival outcome were adjusted for survival factors on multivariable analysis with Cox proportional hazard regression models. Magnitudes of statistical significance were expressed with adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs). All hypotheses were 2-sided, and a p-value of less than 0.05 was considered statistical significant. Statistical Package for Social Sciences (SPSS version 24.0; IBM Corp., Armonk, NY, USA) was used for all the analyses.
Among 6,003 cases identified in the database, 3,196 women younger than 50 years with known oophorectomy status were analyzed. Of those, ovarian conservation at radical hysterectomy was seen in 31 (1.0%; 95% CI=0.6–1.3) cases. Patient demographics are shown in
Table 1. Women who received ovarian conservation were younger compared to those had oophorectomy at surgery (mean age, 37.0 vs. 39.1 years; proportion of age <40 years, 71.0% vs. 49.9%; p=0.033). Early-stage disease was associated with a higher rate of ovarian conservation, and clinical stage IB1 disease had the highest rate of ovarian conservation compared to those in the IB2–IIB stages (1.5% vs. 0.2%–0.4%; p=0.011). Notably, women with adenocarcinoma histology were less likely to receive ovarian conservation at radical hysterectomy in this study population (adenocarcinoma 0.1%, squamous histology 1.3%, and adenosquamous 1.0%; p=0.021). Remaining surgical-pathological factors were not associated with ovarian conservation (all, p>0.050).
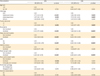
Survival analyses were performed. Median follow-up time among the cases without survival events was 5.4 years. There were 586 recurrences and 355 deaths from cervical cancer during the follow-up. Women who had ovarian conservation at surgery had a similar DFS rate compared to those who had oophorectomy (5-year rates, 75.0% vs. 82.6%, p=0.290). Similarly, CSS was similar between the ovarian conservation group and the oophorectomy group (5-year rates, 87.8% vs. 89.2%, p=0.850). On multivariable analysis controlling for age and other surgical-pathological factors (
Table 2), ovarian conservation was not associated with DFS (aHR=1.85; 95% CI=0.82–4.19; p=0.140) and CSS (aHR=1.22; 95% CI=0.39–3.85; p=0.740) in this study population.
We found that women younger than 50 years with clinical stage IB–IIB cervical cancer rarely undergo ovarian conservation at the time of radical hysterectomy in Japan. This finding is clearly different from what has shown in the US population where the ovarian conservation rates for stage IB disease showed nearly 30-fold higher [
5]. The exact reason for this fairly low rate of ovarian conservation at surgical treatment in Japan is unknown, however, one possibility to explain this association is that surgeons may be concerned for ovarian metastases at the time of radical hysterectomy.
In review of the literature, there is an increased risk of ovarian metastasis with adenocarcinoma histology as compared to squamous type reported in multiple studies [
6789]. Likely reflecting this evidence, women with adenocarcinoma type had a minimal rate of ovarian conservation in this study population (0.1%). However, a recent meta-analysis of 5 studies showed that ovarian recurrence was not observed after ovarian conservation for early-stage (carcinoma in situ [CIS] to stage IIA) cervical adenocarcinoma [
9]. Moreover, a population-based study showed that CSS from early-stage cervical adenocarcinoma was similar between the ovarian conservation group and the oophorectomy group (aHR=0.90; 95% CI=0.50–1.61) [
7]. In our study, there were only 2 women with adenocarcinoma histology who had ovarian conservation, and survival outcome of this subgroup was not assessable. Given that the absolute incidence of ovarian metastasis remains relatively low even in adenocarcinoma type (2.0%–3.7%) [
79], ovarian conservation may need to be considered even in adenocarcinoma histology when there is absence of additional risk factors for ovarian metastasis [
8].
Another theoretical concern for ovarian preservation at the time of surgery is a risk of metachronous ovarian cancer that would otherwise be removed at the time of surgical treatment for cervical cancer. However, unlike endometrial cancer where there is a genetic link between ovarian and endometrial cancer, cervical cancer is generally associated with persistent infection to oncogenic human papillomavirus and genetic susceptibility to ovarian cancer is unlikely. Pelvic irradiation is known to increase the risk of metachronous ovarian cancer in women with cervical cancer as demonstrated in population-based study [
10], however, the absolute risk of this remains low and takes more than decade to develop. In addition, the use of adjuvant radiotherapy with early-stage cervical cancer is historically uncommon in Japan and chemotherapy is more commonly utilized.
Ovarian conservation at surgery may potentially improve overall survival of young women with early-stage cervical cancer due to cardioprotective effects of ovarian hormones [
5]. Although it did not reach statistical significance likely due to short follow-up time, recent population-based study showed a trend towards improved overall survival in young women with stage IB cervical cancer who had ovarian conservation as compared to those who had oophorectomy [
5]. Thus, preservation of the ovary should be beneficial as long as ovarian conservation does not increase mortality from cervical cancer. A society-based strategy will be the key to improve the ovarian conservation rate for young women undergoing surgical treatment in early-stage cervical cancer in Japan.
Figures and Tables
Table 1
Patient demographics

|
Characteristic |
Ovarian conservation (+) (n=31) |
Ovarian conservation (−) (n=3,195) |
p-value |
|
Age (yr) |
37.0±5.2 |
39.1±6.2 |
0.033
|
|
<40 |
22 (71.0) |
1,579 (49.9) |
|
40–49 |
9 (29.0) |
1,586 (50.1) |
|
Clinical stage |
|
|
0.011
|
|
IB1 |
27 (1.5) |
1,818 (98.5) |
|
IB2 |
2 (0.3) |
585 (99.7) |
|
IIA |
1 (0.4) |
247 (99.6) |
|
IIB |
1 (0.2) |
515 (99.8) |
|
Histology |
|
|
0.021
|
|
SCC |
27 (1.3) |
1,974 (98.7) |
|
Adenocarcinoma |
1 (0.1) |
837 (99.9) |
|
Adenosquamous |
3 (1.0) |
311 (99.0) |
|
Others |
0 |
43 (100.0) |
|
Tumor size (cm) |
|
|
0.210 |
|
≤4 |
23 (1.1) |
2,080 (98.9) |
|
>4 |
5 (0.5) |
909 (99.5) |
|
Parametria |
|
|
0.210 |
|
Not involved |
29 (1.1) |
2,678 (98.9) |
|
Involved |
2 (0.4) |
487 (99.6) |
|
Deep stromal invasion |
|
|
0.480 |
|
Not involved |
18 (1.1) |
1,556 (98.9) |
|
Involved |
13 (0.9) |
1,471 (99.1) |
|
LVSI |
|
|
0.720 |
|
No |
13 (0.9) |
1,404 (99.1) |
|
Yes |
18 (1.1) |
1,667 (98.9) |
|
Uterine corpus |
|
|
0.240 |
|
Not involved |
26 (0.9) |
2,832 (99.1) |
|
Involved |
5 (1.5) |
323 (98.5) |
|
Pelvic lymph node |
|
|
0.990 |
|
Not involved |
23 (1.0) |
2,351 (99.0) |
|
Involved |
8 (1.0) |
794 (99.0) |
|
Para-aortic lymph node |
|
|
0.140 |
|
Not involved |
8 (1.8) |
447 (98.2) |
|
Involved |
0 |
60 (100.0) |
|
Clinically not involved |
23 (0.9) |
2,658 (99.1) |
|
Peritoneal cytology |
|
|
0.280 |
|
No malignancy |
27 (1.1) |
2,467 (98.9) |
|
Malignant cells |
4 (0.6) |
693 (99.4) |

Table 2
Multivariable analysis for survival outcomes
|
Characteristic |
DFS |
CSS |
|
HR (95% CI) |
p-value |
HR (95% CI) |
p-value |
|
Age (yr) |
|
|
|
|
|
<40 |
1.00 |
- |
1.00 |
- |
|
40–49 |
0.97 (0.81–1.16) |
0.720 |
0.84 (0.67–1.05) |
0.120 |
|
Histology |
|
|
|
|
|
SCC |
1.00 |
- |
1.00 |
- |
|
Adenocarcinoma |
1.77 (1.43–2.19) |
<0.001
|
1.56 (1.19–2.05) |
0.001
|
|
Adenosquamous |
1.47 (1.10–1.97) |
0.010
|
1.75 (1.25–2.45) |
0.001
|
|
Others |
1.56 (0.80–3.05) |
0.190 |
1.24 (0.55–2.81) |
0.610 |
|
Tumor size (cm) |
|
|
|
|
|
≤4 |
1.00 |
- |
1.00 |
- |
|
>4 |
1.30 (1.06–1.60) |
0.012
|
1.50 (1.17–1.93) |
0.001
|
|
Parametria |
|
|
|
|
|
Not involved |
1.00 |
- |
1.00 |
- |
|
Involved |
2.16 (1.74–2.68) |
<0.001
|
1.96 (1.52–2.54) |
<0.001
|
|
Deep stromal invasion |
|
|
|
|
|
Not involved |
1.00 |
- |
1.00 |
- |
|
Involved |
1.65 (1.28–2.13) |
<0.001
|
2.03 (1.42–2.90) |
<0.001
|
|
LVSI |
|
|
|
|
|
No |
1.00 |
- |
1.00 |
- |
|
Yes |
1.90 (1.46–2.47) |
<0.001
|
2.83 (1.90–4.22) |
<0.001
|
|
Uterine corpus |
|
|
|
|
|
Not involved |
1.00 |
- |
1.00 |
- |
|
Involved |
0.98 (0.77–1.25) |
0.890 |
1.15 (0.87–1.51) |
0.330 |
|
Pelvic lymph node |
|
|
|
|
|
Not involved |
1.00 |
- |
1.00 |
- |
|
Involved |
2.23 (1.81–2.74) |
<0.001
|
2.65 (2.04–3.44) |
<0.001
|
|
Para-aortic lymph node |
|
|
|
|
|
Not involved |
1.00 |
- |
1.00 |
- |
|
Involved |
1.93 (1.22–3.06) |
0.005
|
2.45 (1.48–4.05) |
0.001 |
|
Clinically not involved |
1.07 (0.83–1.39) |
0.590 |
1.11 (0.80–1.54) |
0.550 |
|
Peritoneal cytology |
|
|
|
|
|
No malignancy |
1.00 |
- |
1.00 |
- |
|
Malignant cells |
1.05 (0.85–1.30) |
0.670 |
0.96 (0.75–1.24) |
0.770 |
|
Ovarian conservation |
|
|
|
|
|
No |
1.00 |
- |
1.00 |
- |
|
Yes |
1.85 (0.82–4.19) |
0.140 |
1.22 (0.39–3.85) |
0.740 |

ACKNOWLEDGMENTS
We thank all the Japan Gynecologic Oncology Group (JGOG) institutions participated in this study and the JGOG Cervical Cancer Committee members for administrative work for the study. We also thank Dr. Brendan H. Grubbs for his scientific input to this study.
References
1. Hamashima C, Aoki D, Miyagi E, Saito E, Nakayama T, Sagawa M, et al. The Japanese guideline for cervical cancer screening. Jpn J Clin Oncol. 2010; 40:485–502.
2. Saito T, Katabuchi H. Annual report of the Committee on Gynecologic Oncology, Japan Society of Obstetrics and Gynecology: patient annual report for 2013 and treatment annual report for 2008. J Obstet Gynaecol Res. 2016; 42:1069–1079.
3. Ebina Y, Yaegashi N, Katabuchi H, Nagase S, Udagawa Y, Hachisuga T, et al. Japan Society of Gynecologic Oncology guidelines 2011 for the treatment of uterine cervical cancer. Int J Clin Oncol. 2015; 20:240–248.
4. Mytton J, Evison F, Chilton PJ, Lilford RJ. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017; 356:j372.
5. Matsuo K, Machida H, Shoupe D, Melamed A, Muderspach LI, Roman LD, et al. Ovarian conservation and overall survival in young women with early-stage cervical cancer. Obstet Gynecol. 2017; 129:139–151.
6. Shimada M, Kigawa J, Nishimura R, Yamaguchi S, Kuzuya K, Nakanishi T, et al. Ovarian metastasis in carcinoma of the uterine cervix. Gynecol Oncol. 2006; 101:234–237.
7. Lyu J, Sun T, Tan X. Ovarian preservation in young patients with stage I cervical adenocarcinoma: a surveillance, epidemiology, and end results study. Int J Gynecol Cancer. 2014; 24:1513–1520.
8. Touhami O, Plante M. Should ovaries be removed or not in (early-stage) adenocarcinoma of the uterine cervix: a review. Gynecol Oncol. 2015; 136:384–388.
9. Jiao XB, Hu J, Zhu LR. The safety of ovarian preservation in early-stage adenocarcinoma compared with squamous cell carcinoma of uterine cervix: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2016; 26:1510–1514.
10. Chaturvedi AK, Engels EA, Gilbert ES, Chen BE, Storm H, Lynch CF, et al. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst. 2007; 99:1634–1643.






