Abstract
Objective
Ovarian clear cell carcinoma (CCC) is one of histological subtypes showing poor prognosis due to chemoresistance. The association of autophagy-related proteins and clinical implementation in CCC has not been determined.
Methods
The present study investigated whether expression of autophagy-related protein, light chain 3A (LC3A), was related with prognoses in the patients with CCC using immuno-histochemical stainings, and whether inhibition of autophagy modified the sensitivity to cisplatin in CCC cells in vitro.
Results
High expression of autophagy-related protein, LC3A, was detected in 78 cases (78%) in all CCC cases. The patients with high LC3A expression showed significantly lower response rate to primary chemotherapy (17% vs. 100%, p<0.010), and had worse progression-free survival (PFS) and overall survival (OS) compared with those with LC3A low expression. Furthermore, multivariate analyses revealed that high expression of LC3A was identified as independent worse prognostic factors for PFS and OS. Inhibition of autophagy protein LC3A using hydroxychloroquine (HCQ) increased sensitivity to cisplatin in CCC cells in vitro.
Conclusion
High expression of LC3A proteins was associated with lower response to platinum therapy, leading to worse prognoses in CCC. Although further studies are needed to confirm the results, inhibition of autophagy by HCQ was associated with platinum sensitivity. Autophagy protein LC3A could be a promising target for treatment for CCC.
Go to : 
References
1. Young RC, Decker DG, Wharton JT, Piver MS, Sindelar WF, Edwards BK, et al. Staging laparotomy in early ovarian cancer. JAMA. 1983; 250:3072–6.

3. Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000; 88:2584–9.

4. Takano M, Kikuchi Y, Yaegashi N, Kuzuya K, Ueki M, Tsuda H, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006; 94:1369–74.

5. Miyamoto M, Takano M, Goto T, Kato M, Sasaki N, Tsuda H, et al. Clear cell histology as a poor prognostic factor for advanced epithelial ovarian cancer: a single institutional case series through central pathologic review. J Gynecol Oncol. 2013; 24:37–43.

6. Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004; 6:463–77.
7. Notte A, Leclere L, Michiels C. Autophagy as a mediator of chemotherapy-induced cell death in cancer. Biochem Pharmacol. 2011; 82:427–34.

8. Orfanelli T, Jeong JM, Doulaveris G, Holcomb K, Witkin SS. Involvement of autophagy in cervical, endometrial and ovarian cancer. Int J Cancer. 2014; 135:519–28.

9. Spowart JE, Townsend KN, Huwait H, Eshragh S, West NR, Ries JN, et al. The Autophagy protein LC3A correlates with hypoxia and is a prognostic marker of patient survival in clear cell ovarian cancer. J Pathol. 2012; 228:437–47.

10. Sasa H, Ishii K, Hirata J, Kikuchi Y, Nagata I, Kawai T, et al. Establishment and characterization of a CA125-producing human ovarian clear cell carcinoma cell line. Hum Cell. 1993; 6:279–86.
11. Winter WE 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007; 25:3621–7.

12. Chan JK, Tian C, Monk BJ, Herzog T, Kapp DS, Bell J, et al. Prognostic factors for high-risk early-stage epithelial ovarian cancer: a Gynecologic Oncology Group study. Cancer. 2008; 112:2202–10.

13. Winter WE 3rd, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2008; 26:83–9.

14. Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013; 4:e838.

15. Mabuchi S, Kuroda H, Takahashi R, Sasano T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 2015; 137:173–9.

16. Yamaguchi K, Huang Z, Matsumura N, Mandai M, Okamoto T, Baba T, et al. Epigenetic determinants of ovarian clear cell carcinoma biology. Int J Cancer. 2014; 135:585–97.

17. Wang J, Wu GS. Role of autophagy in cisplatin resistance in ovarian cancer cells. J Biol Chem. 2014; 289:17163–73.

18. Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL, Wu H, et al. NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene. 2012; 31:1055–64.

19. Xiao L, Shi XY, Zhang Y, Zhu Y, Zhu L, Tian W, et al. YAP induces cisplatin resistance through activation of autophagy in human ovarian carcinoma cells. Onco Targets Ther. 2016; 9:1105–14.
Go to : 
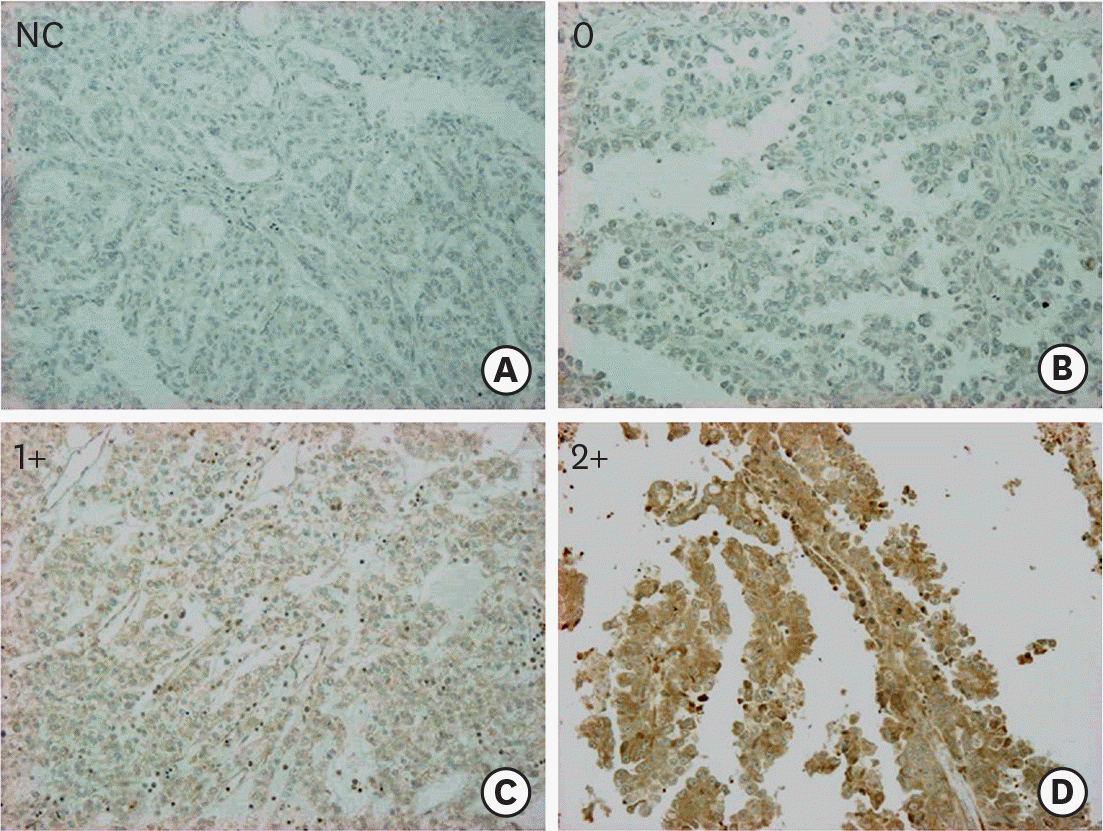 | Fig. 1.Representative IHC stains of LC3A in tissue microarray-based samples of ovarian CCCs (×10). (A) NC, (B) score 0, (C) score 1+, and (D) score 2+. CCC, clear cell carcinoma; IHC, immunohistochemical; LC3A, light chain 3A; NC, negative control. |
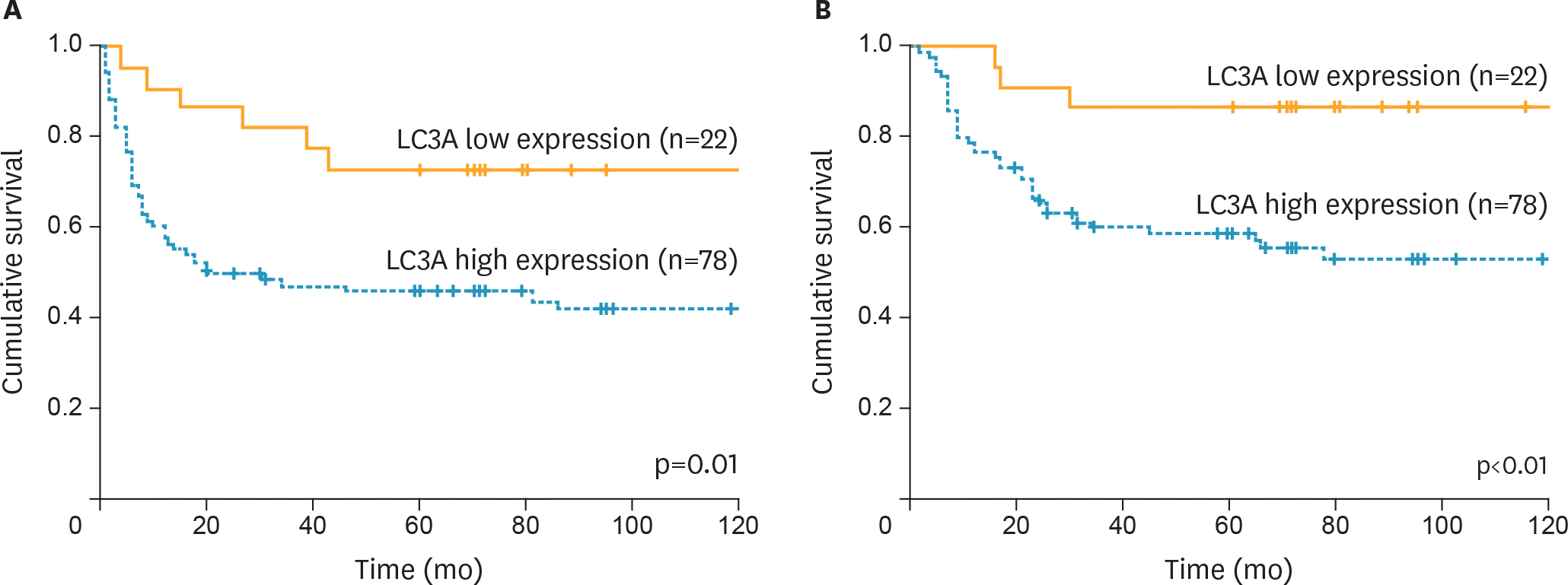 | Fig. 2.PFS and OS of the patients with ovarian CCCs according to LC3A expressions. (A) PFS. (B) OS. CCC, clear cell carcinoma; LC3A, light chain 3A; OS, overall survival; PFS, progression-free survival. |
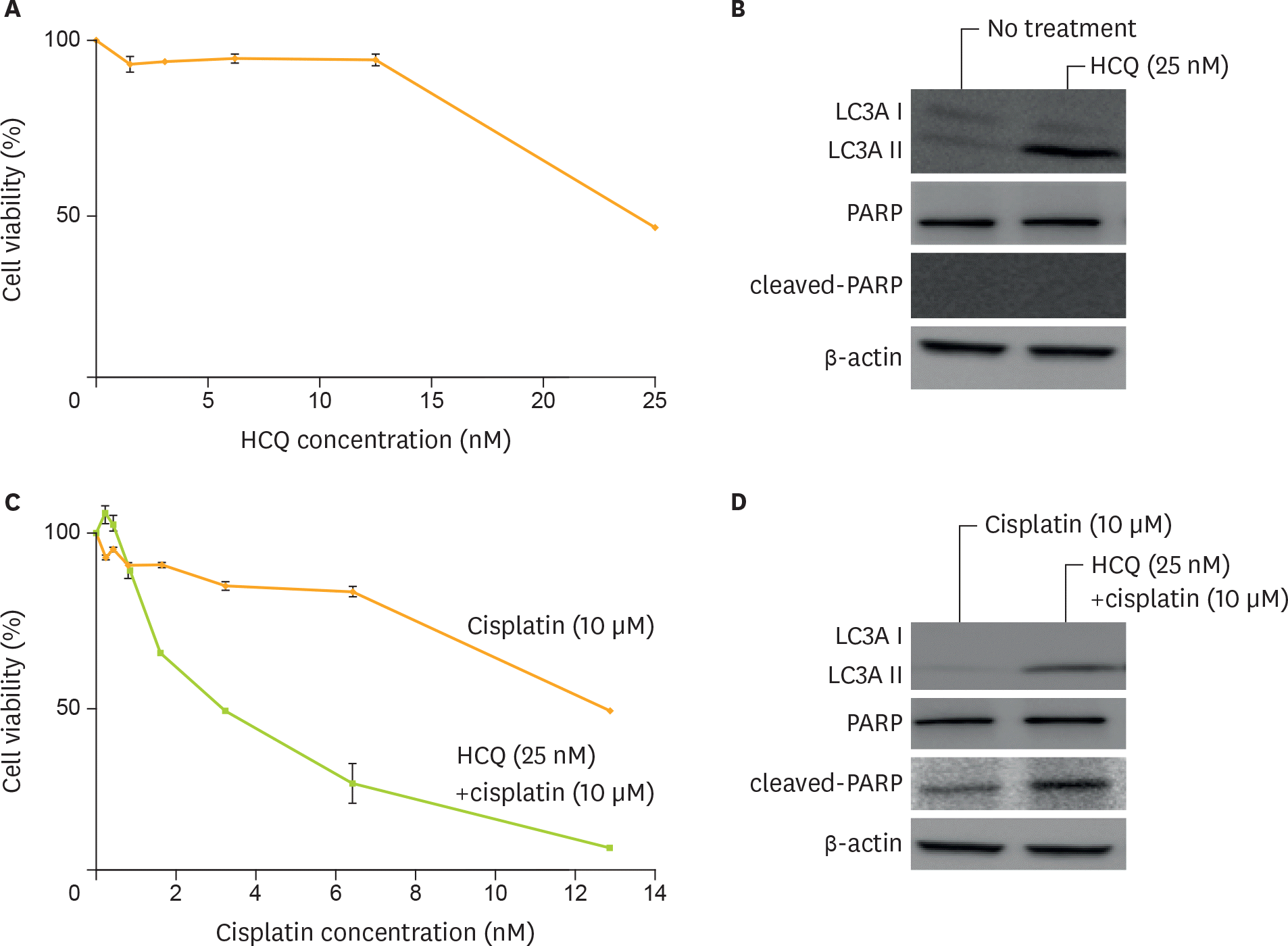 | Fig. 3.Sensitization to cisplatin by autophagy inhibition using HCQ sulfate in ovarian clear cell cancer cell lines. (A) The activity of HCQ as a single agent was measured. (B) Western blotting analysis after treatment by 0 and 25 μ M of HCQ for 24 hours revealed up-regulation of LC3AII, indicating inhibition of autophagy. (C) Cell viability by cisplatin treatment after pre-treatment using 25 μ M of HCQ was measured. (D) Western blotting analysis revealed up-regulation of LC3AII and cleaved-PARP after treatment of 10 nM of cisplatin, with or without 25 μ M of HCQ. Equivalent amounts (10 μ g) of proteins were subjected to SDS-PAGE and blotted with anti-LC3A, anti-PARP, anti-cleaved-PARP, or anti-β-actin antibodies. Cell viability was assessed at 5 days after treatment by MTT assay. HCQ, hydroxychloroquine sulfate; LC3A, light chain 3A; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PARP, polymerase; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis. |
Table 1.
Characteristics of the patients with ovarian CCCs according to LC3A expression levels
Table 2.
Multivariate analyses for PFS and OS in the patients with ovarian CCCs




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download